An abdominal aortic aneurysm (AAA) is a localized dilatation (ballooning) of the abdominal aorta which frequently occurs infra-renally and is superior to the bifurcation of the common iliac arteries (Gilbert et al, 2006) (Figure 1). In similarity with other cardiovascular emergencies, various risk factors including smoking, excessive alcohol consumption, sedentary lifestyle and co-morbidities such as hypertension and diabetes mellitus lead to the onset of atherosclerotic processes which predispose individuals—particularly men aged 60–70—to AAA (Rodin et al, 2003).
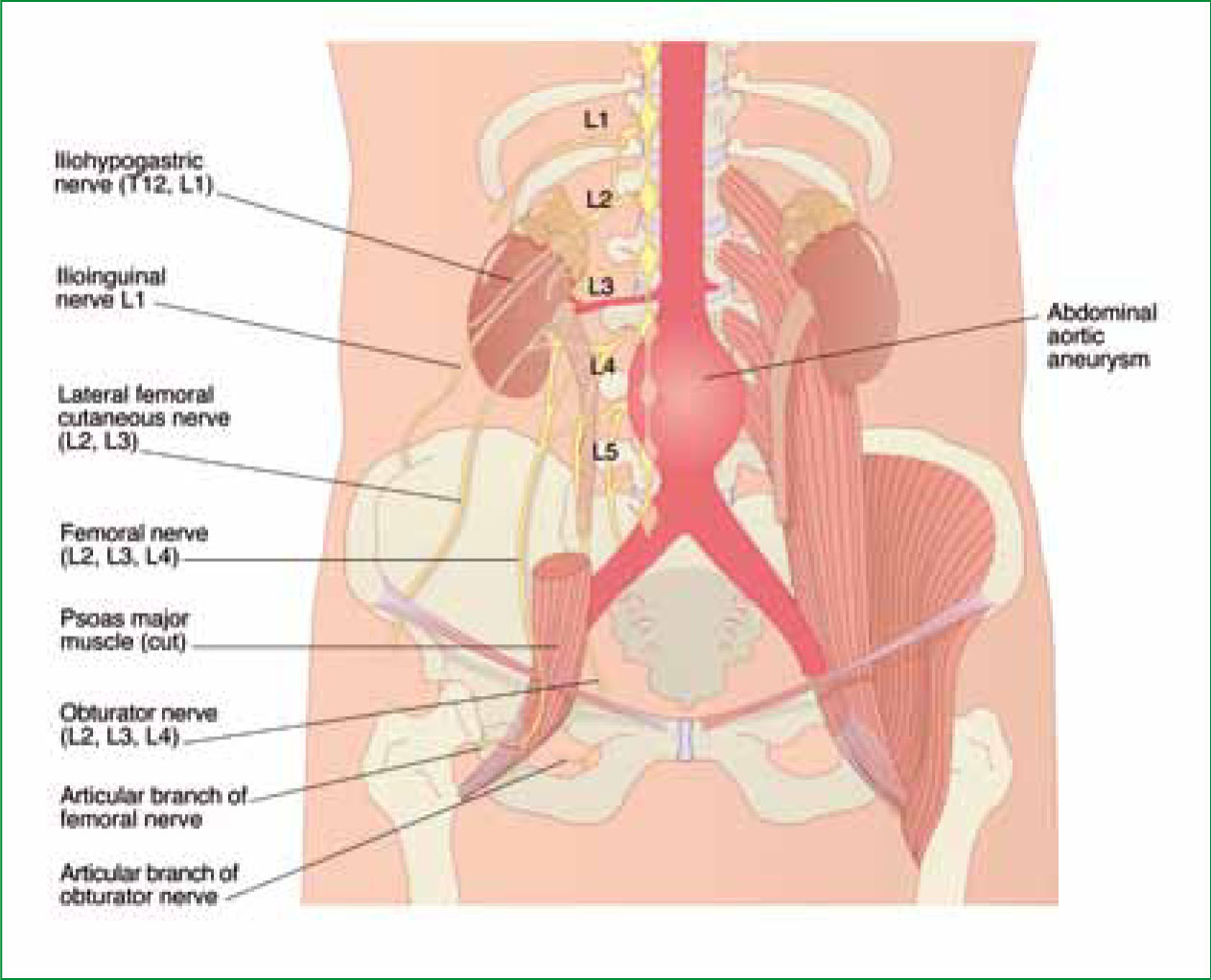
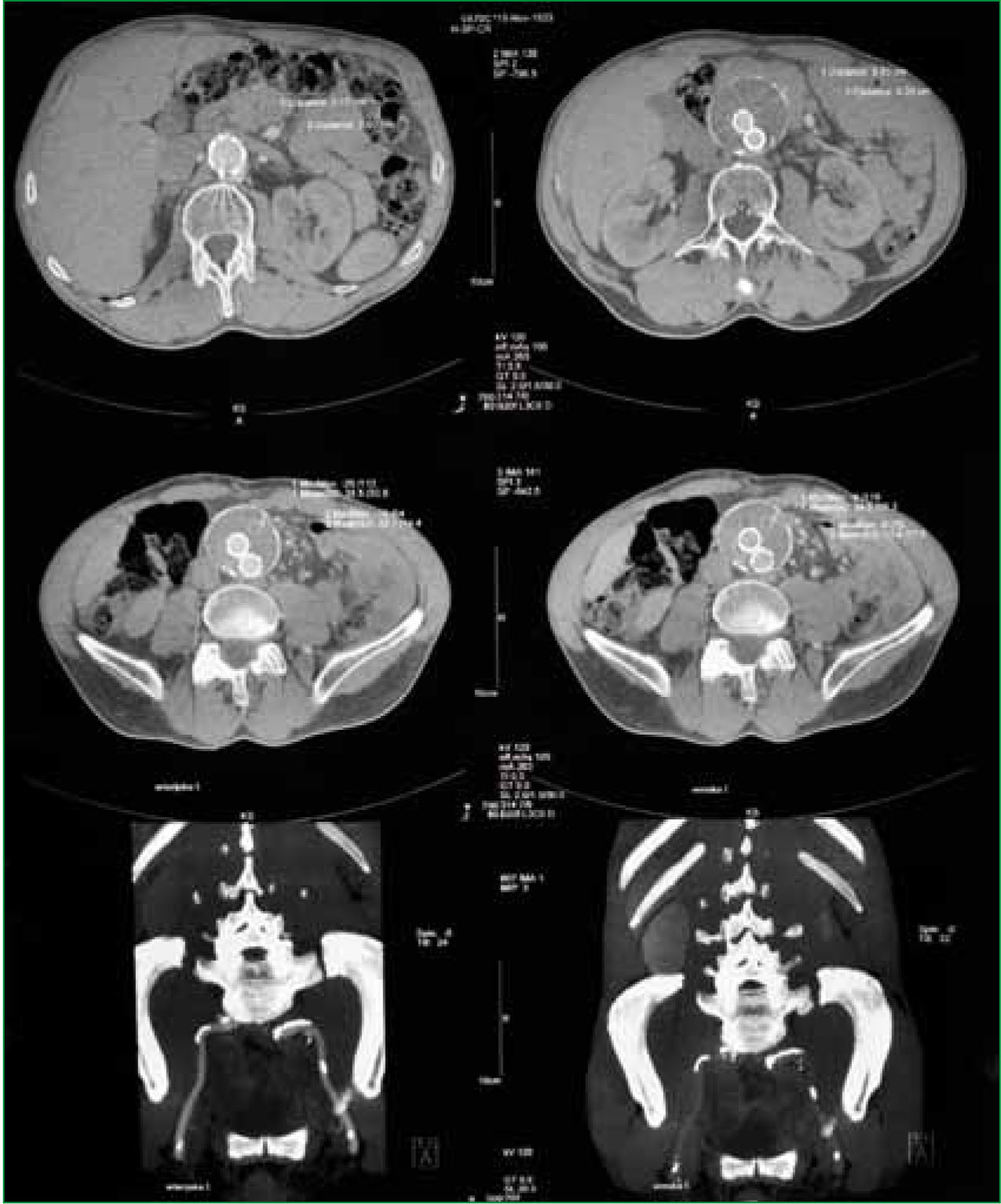
Various pre-existing medical conditions, particularly inherited connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome, increase an individuals risk of developing an AAA. An initial diagnosis is made by physical examination and subsequently confirmed by X-ray, ultrasound or computerized tomography (CT) scanning.
Small, non-dissecting aneurysms are typically monitored and managed conservatively (Lederle et al, 2002). Conservative management focuses on the prevention of expansion and typically involves health and lifestyle advice relating to diet and exercise coupled with preventative pharmacological interventions including the use of ACE inhibitors, statins and beta blockers.
There is some evidence to suggest that the use of propranolol can decrease the rate of expansion (Gadowski et al, 1994). However, a beta blockade will also mask shock-induced tachycardia and this should be considered when paramedic practitioners suspect ruptured AAA in the asymptomatic patient.
Larger aneurysms, and rapidly expanding aneurysms, require surgical repair (Brewster et al, 2003). However, some authors have suggested that even small, non-dissecting aneurysms should be surgically repaired (Hollier et al, 1992) although the evidence from more recent randomized controlled trials suggests that this does not confer any survival advantage (UK Small Aneurysm Trial, 1998; Lederle et al, 2002).
The size at which an aneurysm should be considered suitable for surgical intervention is a source of some debate, however patients presenting with aneurysms greater than 5 cm in diameter would generally be referred for surgical assessment (Lederle et al, 2002). In both cases, AAA are at risk of rupture and this article aims to discuss the initial recognition and prehospital management of the ruptured AAA.
The cardinal signs
The triad of acute abdominal pain coupled with a pulsatile abdominal mass and indications of shock are the cardinal signs of a ruptured AAA (Gilbert et al, 2006). However, such cardinal signs might well be absent in some individuals and a high index of suspicion should be maintained in all individuals and particularly those with predisposing risk factors. The ruptured AAA inevitably leads to hypovolaemic shock and ultimately cardiac arrest. It is for this reason that paramedic practitioners need to consider reversible causes when attending patients in cardiac arrest.
The paramedic practitioner, suspecting a ruptured AAA based on patient history and baseline observations, should perform a focused physical examination which must include inspection and palpation of the exposed abdomen over the bifurcation of the common iliac arteries, above the level of the umbilicus in an attempt to locate an expansile pulsatile abdominal mass.
Similarly, since the ruptured AAA leads to hypovolaemic shock, the AAA patient will often present with a marked tachycardia, profound hypotension, diaphoresis, elevated respiratory rate, increased capilliary refill time, cool peripheries and cyanosis. The presence of flank ecchymoses, referred to as Grey-Turners sign, is particularly worrisome and is indicative of catastrophic retroperitoneal haemorrhage (Pearce, 2009).
Patients might also present with vomiting, haematemesis, lower limb and/or groin pain and neurological deficits. A brachiofemoral pulse delay or asymmetrical and/or absent or diminished femoral, popliteal or pedal pulses are suggestive of a dissecting AAA and can lead to an ischaemic response of the kidneys precipitating acute renal failure, potentially necessitating post-operative dialysis (Tortora, 2005).
The medical literature also refers to the presence of bruits which can occasionally be heard when auscultating with the diaphragm of a stethoscope (Pearce, 2009). The paramedic practitioner, with limited experience of abdominal auscultation, would be making a grave error in delaying transfer to definitive care in order to perform abdominal auscultation on a symptomatic patient. This should not be performed outside of the hospital environment in a patient with a potentially ruptured AAA.
Although the ruptured AAA is a medical emergency, catastrophic haemorrhage results and the subsequent management shares much in common with the prehospital trauma life support model, i.e. initial assessment, immediate correction of life threats and expedient transfer to definitive care (Prehospital Trauma Life Support (PHTLS), 2006). It must be acknowledged that while the insertion of intravenous lines and the administration of fluid therapy and analgesia can be achieved on route, stabilization in the fi eld is not feasible and the emphasis must be on interim measures and rapid conveyance to an appropriate receiving facility coupled with a hospital pre-alert message.
Patients presenting with a ruptured AAA have traditionally been conveyed in the Trendelenberg position (supine with legs elevated above the level of the heart), which was thought to aid autotransfusion through venous return (Baskett, 1990). However, more recent evidence suggests such positioning can have a detrimental effect on ventilatory function by increasing intra-thoracic pressure. Furthermore, symptomatic patients are generally maximally vasoconstricted and Trendelenberg positioning is unlikely to result in significant autotransfusion of visceral organs (Johnson et al, 2002).
Patients should therefore be conveyed in a supine position, with adequate monitoring and oxygen delivery via an appropriate face mask in accordance with pulse oximetry and national guidelines (Joint Royal Colleges Ambulance Liaison Committee (JRCALC), 2006; British Thoracic Society, 2008). Defibrillation pads should be attached, and the paramedic practitioner should prepare to initiate advanced life support in the event of cardiac arrest.
The fluid debate: should it be watered down?
The insertion of intravenous lines and the administration of fluid therapy, particularly where transfer times are short, is a contentious issue and there is limited evidence to suggest that the prehospital administration of intravenous (IV) fluids improves survival in shocked patients (Lewis, 1986; Bickell et al, 1991; Salomone et al, 2005; Solomonov, 2000).
Indeed, some authors have actually suggested that the prophylactic administration of IV fluids in normotensive or hypertensive patients can be detrimental due to the displacement of blood clots and the dilution of platelets and other clotting factors (Bickell et al, 1991; Solomonov et al, 2000; Salomone et al, 2005).
It is universally agreed that transfer to deflnitive care must not be delayed by paramedic practitioners attempting to gain intravascular access and replace fluid volume. If however, large bore intravenous access can be gained on route to the receiving facility, fluid replacement therapy can be initiated and this will frequently involve the use of isotonic crystalloid solutions. Normal saline (0.9%) is used by ambulance services throughout the UK due to its long shelf life, minimal risk of anaphylactic reactions and economic factors.
However, normal saline (0.9%) can only act as volume replacement and even with large bore cannulae in situ, the rate of haemorrhage will likely exceed the rate of fluid replacement (Lewis, 1986). Furthermore, crystalloid solutions are not retained within the intravascular system and will move by osmotic processes into the interstitial spaces, translocation of electrolytes will also occur further impeding the clotting cascade (Bickell et al, 1991; PHTLS, 2006).
Colloid solutions and volume expanders, however, remain in the intravascular system for extended time periods and since they contain electrolytes and proteins such as albumin, they are less likely to disrupt homeostatic processes. Colloid solutions available within the UK and frequently used within the hospital environment include gelatine solutions such as haemaccel, starch solutions such as hetastarch, dextran solutions such as dextran 70, and human plasma components such as 4.5% albumin. Although more expensive than traditional crystalloid solutions and carrying a theoretical risk of inducing anaphylaxis, colloids would make a suitable alternative to crystalloid solutions in the prehospital management of the ruptured AAA.
Pre-warmed fluids should be administered conservatively in conjunction with non-invasive blood pressure monitoring as there is a theoretical risk that a rapid infusion of a fluid challenge could disrupt clot formation (Bickell et al, 1991). Furthermore, rapid infusion will increase preload, the work of the myocardium and subsequently peripheral blood pressure thus increasing the rate of volume loss (Solomonov et al, 2000). Intravenous colloids should be administered in 250 ml aliquots sufficient to maintain a radial pulse and thus cerebral and coronary perfusion pressures.
Failure to effectively manage the shocked AAA patient can have significant consequences post-operatively. Inadequate management of shock in the prehospital environment will lead to hypoperfusion of the kidneys leading to acute tubular necrosis (ATN) and acute renal failure.
Such conditions necessitate post-operative dialysis increasing the time spent in hospital and thus increasing the risk of iatrogenic infections. While the paramedic practitioners primary responsibility is to rapidly identify and expediently convey the patient to an appropriate receiving facility while initiating aggressive supportive measures, it is worth noting that prehospital interventions can impact upon the patient's quality of life post-operatively, should the patient survive to discharge.
Analgesia in the acute abdomen
Acute, severe and constant abdominal pain is frequently the first sign of the ruptured AAA and is sometimes the only presenting feature in the absence of other classical signs such as a pulsatile abdominal mass and indications of shock, which are frequently preterminal signs. Patients presenting with a ruptured AAA will often complain of excruciating abdominal pain, described as a continual ‘tearing pain’ which radiates into their lumbo-sacral region and flanks. Poor management of the ruptured AAA will lead to an increasingly anxious, shocked patient and ultimately cardiac arrest.
Timely and perhaps most crucially, appropriate interventions should be initiated and for the patient presenting with a ruptured AAA this must include, and sometimes be limited to, rapid identification and expedient conveyance. It should be further emphasized that the initiation of fluid therapy and intravenous pain relief must not delay transfer to definitive care and alternative pain relief options including inhaled analgesic agents should be considered.
‘It is universally agreed that transfer to definitive care must not be delayed by paramedic practitioners attempting to gain intravascular access and replace fluid volume’
However, if intravenous access is gained en route to the appropriate receiving facility and fluid therapy has been initiated, pain relief should also be considered. In the absence of contraindications and in conjunction with non-invasive blood pressure monitoring, opiate analgesia would be considered gold standard. Morphine sulphate is a centrally acting opiate which blocks mu receptors, reduces pre-load and creates a sense of euphoria thus reducing patient anxiety.
Similarly, morphine sulphate reduces mean arterial pressure (MAP) thus inducing pharmacological hypotension which is potentially beneficial in the initial prehospital management of the ruptured AAA patient presenting in a hypertensive or normotensive state (Solomonov et al, 2000; Salomone et al, 2005). Pharmacologically induced hypotension will reduce pre-load and thus stress on the cardiovascular system, reducing the risk of clot displacement (Salomone et al, 2005).
The use of ketamine
The use of ketamine for the prehospital management of acute pain associated with medical emergencies is a controversial issue and currently falls outside of UK scope of paramedic practice. However, ketamine is playing an increasingly significant role in the management of prehospital traumatic injuries and various patient group directives exist for its use by critical care practitioners working under the auspices of air ambulance services.
Furthermore, both ketamine and diamorphine are frequently used within hospital for the management of acute pain associated with myocardial infarction. Although the use of ketamine for the management of pain in the patient with a ruptured AAA is beyond the scope of this article, it is worth a brief consideration.
Ketamine is an N-methyl D-aspartate (NMDA) receptor antagonist which acts as an analgesic and anaesthetic agent. The drug delivered intravenously stimulates the cardiovascular system and induces tachycardia and hypertension. Peripheral vasoconstriction leads to an increase in visceral blood supply and thus mean arterial pressure (MAP) increases. It is for this reason that ketamine, in conjunction with midazolam, is often favoured over opiates for the prehospital management of severe pain associated with multi-system trauma and thermal injuries.
Whether pharmacologically induced hypertension is a positive physiological response in the patient with a ruptured AAA is a source of some debate. However, in the profoundly hypotensive patient, its use could potentially be justified. There is limited evidence regarding the use of ketamine for the management of medical emergencies by paramedic practitioners and its use currently lies firmly outside of the scope of practice for most UK paramedics.
In the absence of a robust evidence-base, morphine sulphate must remain the gold standard for the prehospital management of pain associated with a potentially ruptured AAA.
The emergency department is not definitive care
For the patient presenting with a ruptured AAA, definitive care is rapid surgical intervention and not the emergency department. The surgical team should therefore be booked immediately and the emergency department currently initiates supportive measures only, ensuring that ongoing transfer to definitive care is not delayed. The patient is reviewed by a senior emergency physician who will frequently confirm the provisional diagnosis in the absence of X-ray, ultrasound or CT scanning which would further delay transfer to definitive care (Bown et al, 2002). However, many emergency departments will have immediate access to FAST scanning which may be helpful in confirming the diagnosis.
A full blood count including group and save is initiated and the patient will be continually monitored for signs of circulatory collapse. Volume replacement will be continued with whole blood and the patient should be transferred to theatre with minimal delay.
Since the emergency department should not be considered definitive care and since diagnosis within the department often occurs in the absence of scanning, perhaps paramedic practitioners should be in a position to convey patients with a suspected ruptured AAA directly to theatre thus bypassing the emergency department.
There is already a highly successful precedent in many localities for direct admission to cardiac catherterization laboratories for patients presenting with ST-segment elevation myocardial infarction (STEMI), a condition provisionally diagnosed by paramedic practitioners in the prehospital environment based on 12-lead ECG interpretation.
Perhaps, with increases in paramedic education and the continually broadening scope of paramedic practice, the future prehospital management of the ruptured AAA will include a fast-track pathway with specific criteria for the direct admission to definitive care.
Traditionally, repair of the ruptured AAA requires open laproscopic surgery, cross clamping of the aorta and adjacent vessels and the insertion of a stent made from polyethylene terephthalate (PET). Aortic clamping can lead to ischaemic spinal cord injury with associated neurological deficits. Some centres are now performing cerebrospinal fluid drainage resulting in an increase in perfusion pressure within the spinal cord and a reduced risk of ischaemic injury thus improving the patients quality of life post-operatively (Saf et al, 1997).
Endovascular repair of AAA has traditionally been used only to treat stable aneurysms. However, in recent years there has been some success using this method to treat the patient with a ruptured AAA (Dillion et al, 2007; Choong et al, 2010). While the National Institute for Clinical Excellence (NICE) currently advocates open laproscopic repair for the ruptured AAA, it acknowledges that endovascular repair is a potentially viable alternative and is being carried out subject to scientific trials in accordance with local clinical governance (NICE, 2011). Endovascular repair involves the insertion of a synthetic stent through the femoral artery in a procedure similar to primary percutaneous coronary intervention (PPCI).
Conclusion
The ruptured abdominal aortic aneurysm is a time-critical medical emergency with an extremely poor prognosis. Even when recognized early, the ruptured AAA is associated with a mortality rate in excess of 90% (Gilbert, 2006). Definitive care is immediate surgical repair and even in such cases, only about 40% of patients will survive to discharge (Dimick et al, 2002).
The paramedic practitioner has a key role to play in the initial recognition of the ruptured AAA. A thorough working knowledge of the anatomy and physiology and associated signs and symptoms of the ruptured AAA will significantly increase the paramedic practitioners’ ability to identify AAA in the prehospital environment and initiate ongoing treatment.
Paramedic practitioners must acknowledge that definitive care is surgical repair and unnecessary delays on scene attempting to gain intravascular access will deny the patient the opportunity to receive time sensitive surgical interventions. It is a possibility that the future prehospital management of the ruptured AAA might include a fast-track pathway with direct admission to theatre.
Paramedic practitioners must view their role within the context of the multidisciplinary team and acknowledge that rapid identification, expedient transfer to an appropriate receiving facility with the initiation of appropriate prehospital interventions including fiuid replacement therapy and opiate analgesia can provide the patient with the best possible chance of survival with a positive outcome and minimal post-operative complications.
