Digoxin is classed as a cardiac glycoside and has been used for hundreds of years for its positive inotropic effects in heart failure (HF) caused by decreased ventricular contractility (Eade et al, 2013). Cardiac glycosides act by inhibiting the enzyme associated with the sodium potassium pump (adenosine triphosphatase) causing intracellular sodium to increase. As a result, the normal exchange of sodium and calcium is impaired, leading to a reduced removal of calcium from the cardiac muscle cell (Galbraith et al, 2007).
Consequently, more calcium is pumped into the sarcoplasmic reticulum (SR) to be stored. When an action potential excites the cell, the abundant calcium is released from the SR to the myofilaments and increases the force of contraction (Lilly, 2016).
Additionally, cardiac glycosides have a negative chronotropic and dromotropic effect, which are desirable in the therapeutic management of HF and/or rate control in atrial fibrillation (AF). By enhancing the parasympathetic stimulation of the heart, cardiac glycosides decrease impulse generation of the sinoatrial (SA) node and therefore reduce conductivity within the atrioventricular (AV) node (Galbraith et al, 2007; Joint Formulary Committee, 2019). As the conduction velocity between the AV node and ventricles decreases, the interval between atrial and ventricular contraction increases, which slows the ventricular rate and allows more time for the ventricles to fill (Galbraith et al, 2007; Lilly, 2016).
To summarise, digoxin reduces the rate of ventricular stimulation and improves left ventricular emptying to increase cardiac efficiency in patients with HF and/or AF.
Historically, cardiac glycosides were the main treatment for congestive HF; however, they have now been relegated to secondline therapy after angiotensin converting enzyme (ACE) inhibitors and beta-adrenergic antagonists (beta-blockers) (Lilly, 2016). Yet, despite the introduction and common use of these drugs, it is evident that digoxin still has a role in the management of HF and/or AF (Benlmouden and Billaud, 2016).
Therapeutic index
Digoxin has a very narrow therapeutic margin between toxicity and efficacy, which results in a high incidence of digoxin toxicity (Larsen, 2009). To avoid toxicity, digoxin dosage must be adjusted based on patient age, weight and renal function because digoxin is renally excreted (Benlmouden and Billaud, 2016).
The British National Formulary (BNF) reiterates that renal function is the most important determinant of digoxin dosage (Joint Formulary Committee, 2019). Renal impairment may result in a failure to excrete the drug or its metabolites (Bell et al, 2013). Severe impairment can significantly increase the half-life of digoxin to approximately 3.5–5 days (Cheng and Rybak, 2010; Benlmouden and Billaud, 2016). For these reasons, digoxin may accumulate and cause toxicity, especially as its therapeutic index is narrow.
Older patients are assumed to have a degree of renal impairment, as increased age is associated with loss of nephrons within the kidney (Cheng and Rybak, 2010; Bell et al, 2013). On average, glomerular filtration rate (GFR) is expected to decrease by 10 ml/minute every 10 years after the age of 40 (Stevens et al, 2006). Digoxin is excreted by the kidneys with a clearance rate that is proportional to the GFR. Hence, digoxin dose recommendations for any patient with suspected renal dysfunction should be based on the severity of impairment, creatinine clearance and/or GFR (Bell et al, 2013; Benlmouden and Billaud, 2016).
Without the use of blood testing, prehospital clinicians should consider the risk of toxicity by understanding the relationship between poor renal function and digoxin intake. Reducing the dose of digoxin or extending the drug interval may prevent potential adverse effects in these patients (Faull and Lee, 2007). Consequently, the degree of harm from digoxin treatment can potentially be predicted and prevented before prescription/administration.
Role of digoxin in heart failure
Uncertainty remains regarding clinical efficacy of digoxin use in patients with HF despite relevant research stemming from the early 1990s.
In 1995, the Dutch Ibopamine Multicenter Trial (DIMT) highlighted the need for pharmacological intervention even at the early stages of chronic HF because of the rate of disease progression (Van Veldhuisen et al, 1995). The DIMT and the Prospective Randomized Study of Ventricular Failure and the Efficacy of Digoxin (PROVED) trial had different methods; however, both concluded that once-daily digoxin (0.25 mg and 0.375 mg respectively) had a beneficial outcome on exercise tolerance, and was effective in reducing neurohumoral activation and mean heart rate in comparison with the placebo groups (Uretsky et al, 1993; Van Veldhuisen et al, 1995). The DIMT also found that digoxin (but not ibopamine) resulted in a significantly increased exercise tolerance after 6 months compared with placebo.
The Randomised Assessment of Digoxin on Inhibitors of Angiotensin-Converting Enzyme (RADIANCE) study showed that patients with HF who swapped digoxin for placebo in this randomised controlled trial (RCT) experienced a worsening in HF status and decline in maximal exercise tolerance, despite remaining on the ACE inhibitors and diuretics (Packer et al, 1993; Konstantinou et al, 2016). These studies identified the role of digoxin as monotherapy for HF and these earlier findings encouraged the biggest digoxin-centred RCT to date (Hothi et al, 2013).
In 1997, the Digitalis Investigation Group (DIG, 1997) presented the findings of a large, randomised, double-blind, placebo-controlled trial of digoxin in the treatment of HF. This American trial involved 7788 patients with a left ventricular ejection fraction less than 0.45. The intervention arm received 0.25 mg of digoxin per day and the control arm a placebo drug. Although mortality in both groups was equal (35%), the death rate attributable to HF was slightly less in the digoxin group. Hospital admissions for worsening HF were also lower in these patients. However, there were more deaths from other cardiovascular events such as strokes and arrhythmias in those taking digoxin than in the controls (Larsen, 2009).
Furthermore, a post-hoc analysis of this trial found that patients with a serum digoxin concentration (SDC) of >1.2 ng/ml had an 11.8% higher absolute mortality rate than those receiving placebo (Ouyang et al, 2015). Overall, the trial shows that digoxin successfully reduces hospitalisation from HF; however, the side effects of cardiovascular events and increased SDC equate to the same mortality as those receiving placebo for the same disease (Adams et al, 2014).
The generalisability of these findings is limited for today's population because polypharmacy is now common in the management of HF. The DIG study was performed before routine use of ACE inhibitors, beta-blockers and diuretics, which are now standard therapeutic treatment for patients with HF (National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand, 2011; Hothi et al, 2013). Digoxin may be beneficial in patients with HF in conjunction with newer medication to counteract its adverse effects.
The therapeutic algorithm in the European Society of Cardiology (ESC) guidelines for patients with symptomatic heart failure reduced ejection fraction (HFrEF) shows digoxin as a last-resort therapy to be considered only when a patient with symptomatic HFrEF has been treated with ACE inhibitors, beta-blockers and mineralocorticoid receptor antagonists (Ponikowski et al, 2016). If symptoms remain resistant, the ACE inhibitor can be replaced with an angiotensin II receptor blocker neprilysin inhibitor (ARNI) if tolerated (Ponikowski et al, 2016). If not, heart rate can be managed with ivabradine (<70 beats per minute (bpm)) or cardiac resynchronisation therapy (>130 bpm) (Ponikowski et al, 2016). Only if resistant symptoms remain would the patient be considered for digoxin, implanted devices or heart transplantation (Ponikowski et al, 2016). Intravenous digoxin has been shown to have positive neurohumoral effects in patients with severe HF and elevated left ventricular pressures by reducing cardiac norepinephrine release, which, in turn, increases ejection fraction (Konstantinou et al, 2016).
Supporting this, the DIG (1997) trial investigators reported that digoxin was most effective at reducing mortality and reducing hospitalisation resulting from HF, in patients with a higher New York Heart Association (NYHA) class of HF, enlarged hearts and reduced ejection fraction. Interestingly, digoxin use in patients with symptomatic HFrEF is given only a class 2b recommendation by the ESC as its usefulness and efficacy is less well established by evidence or opinion (Ponikowski et al, 2016). The DIG trial continues to deserve comment, but most authors identify the need for a contemporary RCT to support higher-quality evidence-based practice. Perhaps a lack of research—rather than the drug itself—is the reason for the low classification of recommendation and the ESC guidelines recommending the drug only when all else fails.
Role of digoxin in AF
Current American Heart Association guidelines suggest that digoxin is an acceptable choice for ventricular rate control in AF, yet preferentially recommend a beta-blocker as first-line treatment (Eade et al, 2013; Lilly, 2016). Digoxin is used in patients who cannot tolerate beta-blockers; it therefore remains indispensable as a secondline therapy in these patients for its negative dromotropic and chronotropic effects (Eade et al, 2013).
One trial that supports this recommendation looked at the effects of digoxin in patients with AF. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trail in 2004 assessed mortality in four rate-control drugs, including digoxin. Gheorghiade et al (2013) published a more recent analysis of the study, with a discussion that focused on the use of digoxin in patients with paroxysmal AF and persistent AF (with or without HF). This found that digoxin had no association with increased mortality, hospitalisation or incident non-fatal arrhythmias, either as monotherapy or in combination with other rate-control drugs.
However, Ziff et al (2015) reviewed three post-hoc analyses of the same dataset from the AFFIRM trial, each of which drew different conclusions on non-randomised prescription of digoxin, based on how the authors chose to adjust the data. This only adds to the confusion that physicians must experience when attempting to provide evidence-based practice. To add to the debate, the TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, which ran over 2004–2008, showed that digoxin increased risk of death in patients with new onset, non-valvular AF, independent of drug adherence, kidney function, cardiovascular comorbidities and concomitant therapies (Turakhia et al, 2014). Overall, a meta-analysis of 11 observational studies by Ouyang et al (2015), including the AFFIRM Trial and TREAT-AF studies, found digoxin use was associated with greater risk for mortality in patients with AF, regardless of concomitant heart failure.
Overview of the role of digoxin in HF and/or AF
Ziff et al (2015) carried out a large systematic review of the data collected from 1960–2014 regarding digoxin in patients with HF and/or AF. Despite what appears to be a thorough systematic review, its heterogeneity is deemed high overall because of the variation in methodology among the 52 studies reviewed. However, the heterogeneity is nonexistent (I2=0%) in the forest plot (within this review) of RCTs measuring all-cause mortality of digoxin. It demonstrates that the DIG trial had a very narrow 95% confidence interval (CI) (0.93–1.06) between higher mortality with control versus higher mortality with digoxin respectively. However, this includes the value 1 (shown by the line of no difference), which means the results are not statistically significant. Given disparity, the results of the DIG trial for all-cause mortality in people with HF are not clinically significant either (DIG, 1997; Ziff et al, 2015).
When discussing digoxin use in patients with AF only, Ziff et al (2015) acknowledges that there is still much discrepancy between the findings in contemporary post-hoc analyses, cohort studies and observational studies when assessing mortality risk. Therefore, digoxin must continue to be considered as a treatment option to achieve heart-rate control in patients with AF until proved to be redundant.
It has also been highlighted that the only data assessing mortality risk and hospital admissions connected to AF and digoxin use was collected from observational studies. These data have been criticised for the potential of bias within the methodology, so the aforementioned effects on mortality and admission remain unknown.
Monitoring SDC1
After weighing up the available evidence and using clinical judgment, clinicians continue to prescribe digoxin for HF and/or AF management as per guidelines (Joint Formulary Committee, 2019). Once on the drug, each patient is at risk of digoxin toxicity owing to its narrow therapeutic index. SDC is a measurement of digoxin in the plasma and can be useful to check compliance, adapt dosage or confirm toxicity. A meta-analysis of 11 digoxin-centred studies revealed that an increased SDC is a critical factor for predisposing patients with AF to an increased mortality risk (Ouyang et al, 2015). Although an important, SDC has commonly been omitted from previous digoxin-related studies; this may be owing to time constraints or funding limits, or the importance of SDC measurements may be overlooked. Knowing the SDC throughout treatment would allow clinicians to change dosages to fit the therapeutic window and therefore prevent toxicity.
The target SDC is extremely variable depending on which study is read, often in a range of 0.5–2.0 ng/ml (Goldberger and Goldberger, 2012). In 2010, the Heart Failure Society of America guidelines recommended a target SDC of <1 ng/ml as a result of the success seen in mortality of patients with lower SDCs and the increased occurrence of toxicity recorded at an SDC of between 1 ng/ml and 2 ng/ml (Lindenfeld et al, 2010). Patients with AF are assigned a higher acceptable average target SDC. It must be acknowledged that toxicity can occur even within the target SDC range, and the therapeutic window will be different for each individual (Lindenfeld et al, 2010).
This may be the reason that the National Institute for Health and Care Excellence (NICE, 2016) guidelines recommend an SDC measurement only to confirm the clinical impression or suspicion of digoxin toxicity, to be recorded 8–12 hours after the last dose of digoxin (NICE, 2016). The indication for SDC monitoring is based on overall patient condition with a special consideration and lower monitoring thresholds for patients with renal impairment.
One study analysing more than 120 000 patients undergoing haemodialysis reported an greater rate of 28% if the patient was taking digoxin (Chan et al, 2010). This was partially attributed to the high prevalence of hypokalaemia within this group, which is known to increase the toxicity of digoxin (Goldberger and Goldberger, 2012). Regular monitoring of potassium and SDC in these high-risk patients seems a simple way to detect early digoxin accumulation and avoid toxicity and its symptoms of arrhythmias, nausea, abdominal pain and visual disturbance (Goldberger and Goldberger, 2012; Kirilmaz et al, 2012). These symptoms could be recognised by prehospital clinicians as early signs of digoxin accumulation, and highlight the need for a change in dosage. It could be viewed that the harmful effects of digoxin can be prevented as an alternative to not using digoxin at all.
Pro-arrhythmic effects of digoxin
Patients with digoxin toxicity are at risk of pro-arrhythmic effects (Limon et al, 2016). Acute digoxin toxicity may present with arrhythmias caused by decreased AV conduction and/or increased automaticity (Dawson and Buckley, 2016). These arrhythmias result from increased intracellular calcium, which causes a transient inward depolarising current and triggers abnormal activity within the myocardium (Konstantinou et al, 2016). Electrocardiogram (ECG) changes can vary between infrequent premature ventricular contractions to complete AV blocks, ventricular tachycardias (bidirectional in severe toxicity) and ventricular fibrillation (Kirilmaz et al, 2012; Dawson and Buckley, 2016).
Acute toxicity arrhythmias are caused by the initial loading doses of digoxin or an accidental overdose of digoxin, further complicated in patients with renal failure and prone to hypo- or hyperkalaemia. These pro-arrhythmic effects raise questions over the safety of long-term digoxin use. Long-term users with digoxin toxicity may present with bradyarrhythmias—most likely AF with a slow ventricular rate as a result of its initially desirable negative dromotropic and chronotropic effects (Limon et al, 2016). This is closely linked and prevalent in those who may experience a decrease in potassium stores because of diuretic treatment and secondary hyperaldosteronism in patients with HF (Limon et al, 2016). This complicates prescription of digoxin further and clinicians may favour alternative medication to balancing diuretic treatment with cardiac glycosides in patients with HF to avoid toxicity, hypokalaemia and arrhythmias.
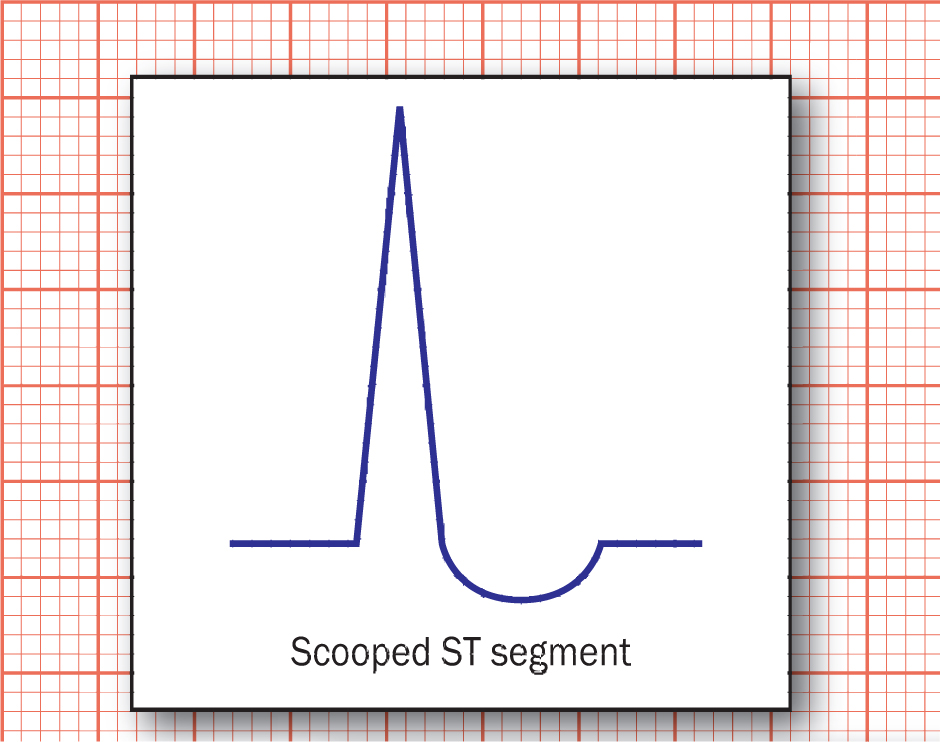
Another common ECG abnormality in long-term digoxin users is the scooped appearance of the ST segment (Figure 1). This is caused by the shortening of ventricular action potential duration, which in turn shortens the QT segment, fusing the ST segment and the T wave as a result (Stucky and Goldberger, 2015). Importantly for prehospital clinicians to note, a scooped ST segment does not equate to toxicity or harm and may be a normal finding in patients on digoxin.
Thromboembolic risks of digoxin
In addition to generating arrhythmias, there is a greater incidence of thromboembolic events seen in patients with AF who are taking digoxin (Ouyang et al, 2015). Digoxin has been shown to increase platelet and endothelial activation as a result of increased intracellular calcium (Chang et al, 2013).
A large-scale, population-based study in Asia showed digoxin-treated patients with AF had a 10% overall increase in stroke incidence over a 10-year follow-up period (Chang et al, 2013). It was recognised that only 23.9% of the sample group were taking warfarin compared with higher percentages of anticoagulant use in similar studies assessing thromboembolic risk, hence the higher prevalence of stroke in this particular study. Regardless of anticoagulant treatment, this study showed that, although all patients with AF are at risk of stroke, those taking digoxin are at a statistically significant higher risk than those who are not (hazard ratio 1.44; CI 95%, 1.22–1.69). It must be recognised, however, that patients with more advanced HF, interval hospitalisation or more recalcitrant AF are more likely to be prescribed digoxin, which is associated with worse clinical outcomes overall (Chang et al, 2013; Dardas and Levy, 2015).
Arrhythmias and thromboembolic events are life-threatening adverse effects of digoxin, which undoubtedly cause harm and may further discourage its prescription/administration.
Conclusion
When treating patients with digoxin, clinicians experience difficulty in attempting to base their practice upon the currently available evidence. There is a lack of robust contemporary research regarding digoxin therapy in conjunction with today's standard medications, or comparing digoxin with alternative management for HF and/or AF. This is recognised as a limitation to drawing conclusions as even the references in this review span more than 20 years, with little evidence to support its use in today's HF and/or AF population.
Hothi et al (2013) state that it would be desirable yet unrealistic to expect multiple future RCTs to assess digoxin usage in such diverse subgroups of patients. Therefore, without clear guidance, it remains each clinician's decision to monitor the results of digoxin in each individual case and manage the results accordingly. The risks are evident and inclusive of arrhythmias, strokes, toxicity and potentially worsening outcomes. However, adverse effects may be predictable in vulnerable patients such as those with renal impairment, and therefore preventable if the prescriber is well informed.
Equally, if prehospital clinicians have an understanding of the pharmacokinetics, the signs and symptoms of early digoxin accumulation, ECG changes in toxicity and potential for stroke, further harm may be prevented. The negative chronotropic, negative dromotropic and positive inotropic effects of digoxin are undeniably desirable in patients with HF and/or AF and, despite controversy, have proven to reduce hospital admissions in those with severe HF. In conclusion, it would be too bold of a statement, and an unsupported one at that, to say that digoxin was a categorically harmful medication when it has shown positive outcomes in the past for the treatment of HF and/or AF.

