Although a common condition, the mechanisms of diabetes are not always simple to understand, and requires concentration as well as an appreciation of multiple body systems. This guide will hopefully further your knowledge of the physiology and help you understand why diabetes and its complications present as a particular set of symptoms.
Diabetes mellitus (DM) is a common condition stemming from the body halting the production of insulin from beta cells in the islets of Langerhans regions of the pancreas (type 1 diabetes), or the way the body reacts to the insulin the body produces, forming a resistance (type 2 diabetes).A lack of insulin means it is unable to inhibit the release of glucagon from the pancreas, which stimulates the liver to break down stored glycogen into glucose, thus raising the blood sugar level at the expense of storing fat.
Glucagon normally releases only when blood sugar levels are too low, using stored energy reserves to maintain a healthy blood glucose level (between 4 mmol/l and 5.9 mmol/l without eating, <7.8 mmol/l postprandial), which is important in the normal function of the brain that depends entirely on glucose in normal physiology. This glycogen that has already been stored will have been mediated by insulin in the first place, and its action on liver, musculoskeletal tissue and adipocytes (fat cells) is what encourages the uptake of blood glucose after meals to keep in reserve. With insulin and glucagon working symbiotically, we are able to regulate our blood glucose effectively. However, when the system is disrupted in DM, problems start to occur, with symptoms related to the pathophysiology behind DM clinically evident.
| Gestational diabetes: occurs in pregnancy. Often predicts the development of type 2 diabetes mellitus. |
| Congenital diabetes: occurs due to genetic defects of insulin secretion. |
| Steroid-induced diabetes: occurs as a result of high doses of glucocorticoids. |
| Diabetes related to cystic fibrosis: the buildup of pancreatic secretions damages insulin-producing cells. |
What happens if you have no insulin?
Before 1922, and the work of Nobel Prize winning Canadian medics Dr Frederick Banting and Charles Best (still a medical student at the time), developing diabetes in childhood was akin to a death sentence. It was described by ancient physician Hippocrates as a short, disgusting and painful condition (Medvei, 1993) due to the rapid dehydration, starvation, pain, and coma leading to death that occurred in type 1 DM.
Polyuria (massive urine production) occurs because the increasing amounts of glucose unregulated in the blood stream shifts the osmotic balance of the blood to the point that when the kidneys filter the blood, less of it is returned at the distal end of the nephron (the structure that filters blood in the kidneys). This means water, and important electrolytes, such as potassium and sodium, are being taken out of the system and no longer returned by the kidney, causing polydipsia (increased thirst), a sensation we feel at just 1% dehydration.
Fortunately, glucose is also excreted along with the other products when it displays high blood levels, but not in significant enough quantities as to correct the problem. Characteristically, patients developing diabetes drink large quantities of water while urinating large quantities of sweet-smelling urine, frequently throughout the day and night. There is an inability to keep up with the vast amounts of water being excreted, which means a lack of insulin also leads to dehydration.
Insulin is released when blood sugar levels rise after a meal. All excess glucose from carbohydrates that are unused by cells for energy are encouraged to be stored as glycogen by a process known as glycogenesis. This is stored as emergency reserves for the body to use in starvation, and release is stimulated by glucagon.
The lack of insulin and subsequent high levels of glucagon lead to a breakdown of fat and muscle tissue glycogen, a large molecule containing hundreds of chains of fatty acids. The body believes that food is scarce and at risk of starvation. Glycogen’s fatty acids are released and converted in the liver. These fatty acids eventually yield FADH2, NADH and acetyl coenzyme A, which are used in the Krebs cycle of intra-cellular energy production. This process is used in cells to continue normal function from energy reserves or normal dietary intake. The brain exclusively uses glucose in normal life, not the products of stored energy breakdown as described above, which can lead to a faint feeling in starvation.
However, unlike in normal function, ketone bodies are produced as a by-product of the fatty acid synthesis. This isn’t normally a problem because the body does not have to break down the carbohydrate glucose that is ideally available to use as energy. These ketone body by-products do have a use, as after a few days starvation they will begin to be taken up as an energy source by the brain and heart to keep a person alive. Until then they are excreted (Table 2).
| Ketones are acidic and can unbalance the pH of the blood. |
| Ketones cannot be converted back into acetyl coenzyme A (acetyl-CoA) and therefore have to be metabolised or excreted within a few hours. This causes a continual process of breakdown in diabetes, even if there is minimal energy expenditure by the patient. |
| Acetone is a by-product, along with CO2 of fatty acid breakdown. It is produced in small quantities in the liver and excreted in respiration and urination. |
| Acetoacetic acid is released when ketones are being used in the production of energy for use in the brain in starvation. |
| beta-Hydroxybutyric acid is used by the brain as an energy source in low-glucose states, and is a by-product of the production of the useful metabolic enzyme acetyl-CoA, which is used in cells as energy through Kreb’s Cycle. |
| Ketone bodies are detected by using a nitroprusside dipstick, which turns from pink to purple in the presence of ketones. |
We can use the fact that ketone bodies are released specifically in the breakdown of fats to detect diabetes. One type of ketone body (acetone) is excreted by the kidney in this process, and is detectable in the urine as ‘ketouria’. A urinalysis positive for this will indicate a starved or diabetic state. Ketones are also excreted in respiration, so a sweet, fruity smell of acetone on a patient’s breath may also be present. Ketones may smell like nail polish remover, because that is one of the domestic uses of acetone.
In the meantime, the normal blood glucose is not being taken into cells and used as energy because insulin isn’t encouraging its uptake. The cells are blind to the presence of glucose and instead use the synthesised fatty acids and ketone bodies to stay alive. The kidney will excrete glucose in detectable, high levels that can be seen in urinalysis, due to the osmotic imbalance discussed earlier. This is called glycosuria. Normally, the nephrons in the kidney reabsorb glucose as it filters blood in the proximal tubule, but at high levels it will be allowed to release through the urine, in an attempt to counter the osmotic imbalance that leads to polyuria.
These previously described problems in diabetes are all unpleasant and distressing, but due to the acidic nature of ketones, the most acute problem becomes evident with a shift in the pH of blood. Blood needs to have a pH between 7.35–7.45 to be compatible in life, and this metabolic acidosis causes a series of symptoms that can quickly lead to death. This will be discussed in the next section.
An undiagnosed diabetic can present acutely, more often in type 1 DM than type 2. Remembering that in type 1 diabetes insulin production may have halted completely, all the above processes are happening, which can appreciably lead to serious illness in a very short period of time. People with type 2 diabetes are not always as acutely affected by their insulin levels as they still have limited function, which is more likely to lead to less specific symptoms such as fatigue and headaches from excessively high blood glucose over a longer period of time, or may only be picked up during routine checkups without any symptoms presented at all.
Diabetic ketoacidosis
Understanding the physiology of diabetes makes it possible to appreciate the acute complications of having a high blood sugar level. If blood sugar is high, then insulin has not been administered effectively or is not available in the body of a person with type 1 diabetes (or rarely some people with type 2 diabetes). This means that, due to the symbiotic nature of insulin and glucagon, glucagon has instead been released in high levels, and fatty acid breakdown with subsequent production of acidic ketone bodies is occurring.
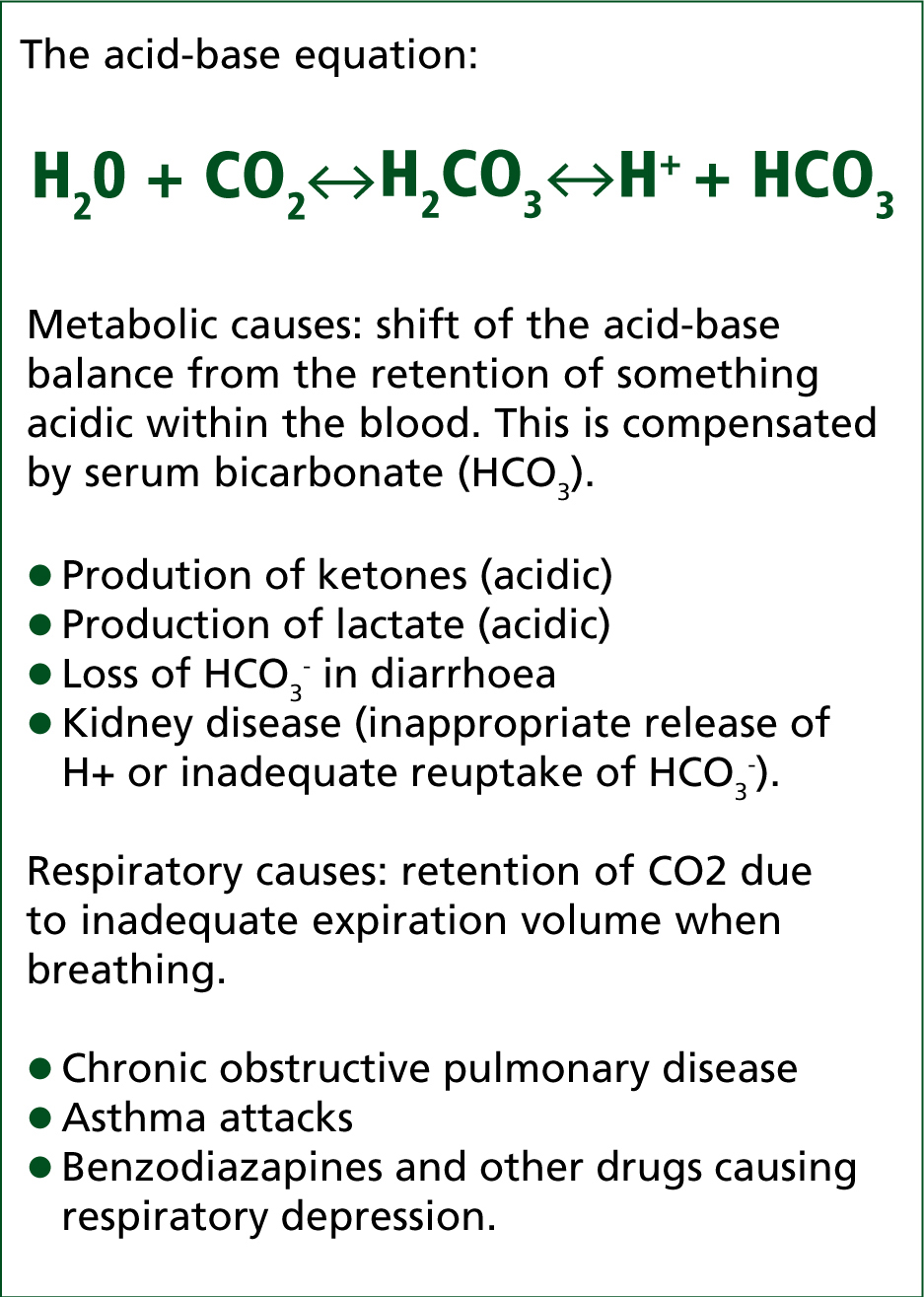
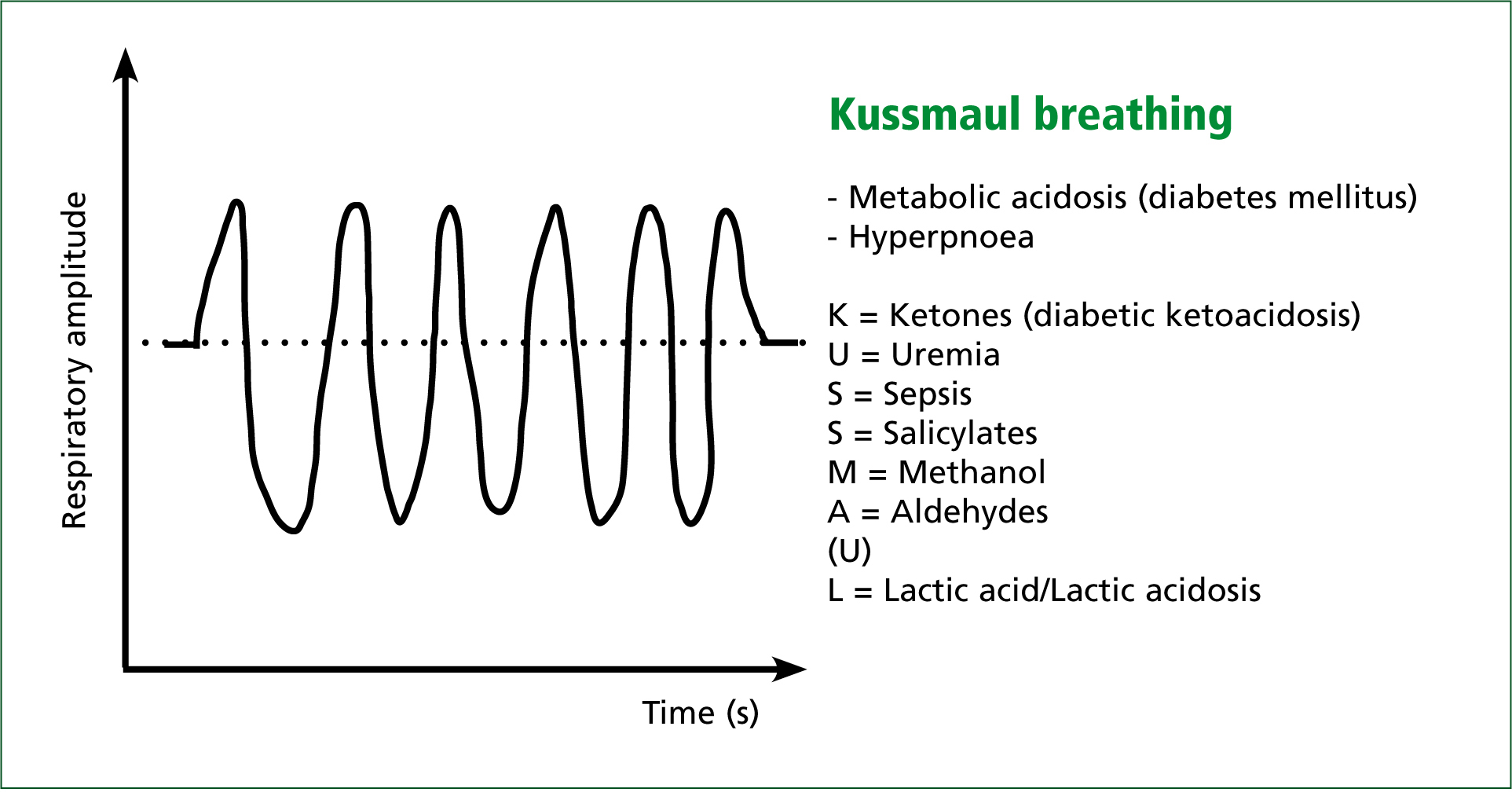
The body tries to correct the acidosis by the buffering system (Figure 1), but serum bicarbonate is quickly depleted (see Figure 1) and instead the expiration of acidic CO2 is attempted, producing a distinctive ‘Kussmaul’ respiration (Figure 2). This merely rids carbon dioxide as compensation for the effect of high levels of acidic ketones in the blood, without doing anything to specifically rid the body of them. The kidneys also increase uptake of bicarbonate (HCO3-) and increase excretion of hydrogen ions (H+).
At the same time the body is dehydrating, with insufficient fluid intake and increased urine output associated with high blood glucose levels. This is shown by a low blood pressure and decreased level of consciousness, as blood isn’t perfusing the vital organs.
Urine output may progressively decrease or cease at this point, as the kidneys aren’t receiving enough blood to filter any products. This is known as hypovolemic shock and is a serious, life-threatening problem. High creatinine and urea levels in the blood are indicative of the condition.
| Mild: blood pH between 7.25 and 7.30, serum bicarbonate decreased to 15–18 mmol/l (normally >20); patient still alert. |
| Moderate: pH 7.00–7.25, serum bicarbonate 10–15 mmol/l, patient may be drowsy. |
| Severe: pH below 7.00, serum bicarbonate <10 mmol/l, loss of consciousness and coma. |
Patients vomit and experience abdominal pain as the blood becomes increasingly acidotic. Vomiting releases the acidic H+ ion from the stomach, which is a compensation for the acidosis, but this is not significant and instead simply leads to further dehydration. The pain may be caused by ischaemia due to hypoperfusion.
Remembering that potassium was one of the electrolytes lost with the other fluids, this must be stabilised in diabetic ketoacidosis. Insulin normally shifts potassium into cells, but in the absence of insulin, potassium stays in the blood, and is subsequently lost in the kidneys. Low levels of potassium (hypokalaemia) can lead to cardiac irregularities, so this will need to be monitored and corrected or it may lead to death.
Acidosis has a direct effect on the brain, causing malfunction and subsequent disorientation and coma. Circulating volume has to be increased and ketone levels reduced to prevent neurological symptoms in acidosis (Rehncrona, 1985). Hypoperfusion of the brain due to a drop in blood pressure can also alter consciousness. In the same way the kidneys are not getting enough blood, the brain needs adequate blood pressure to maintain consciousness.
Diabetic ketoacidosis is simply a lack of insulin available to deal with high blood glucose, but can sometimes occur despite good management of the condition. This is because in some states more insulin is required for management. These include recurrent infections, pneumonia, pregnancy, strokes and myocardial infarctions, as well as pregnancy. Correction of diabetic ketoacidosis includes the carefully controlled administration of insulin, hypoperfusion from hypovolaemic shock, due to dehydration, and loss of potassium due to high blood glucose. These are the dangerous events that occur in ketoacidosis, along with acidity interfering with brain function, which can lead to coma.
Despite many of the complications stemming from a lack of fluid in the circulating volume, a build-up of fluid in other compartments can occur, e.g. the brain, causing cerebral oedema, and in the lungs, causing respiratory distress.
Cerebral oedema occurs in ketoacidosis because the brain readily absorbs the fluids, which are low in the electrolytes that would normally allow too much fluid to be absorbed at any one time. In the same way the kidney wastes a lot of the fluid by taking it and not giving it back, the brain does something similar. This is especially a risk during treatment when intravenous fluids are given, and care is taken to replace electrolytes and slowly reintroduce fluids into the circulating volume. If dispensed too fast, the brain may take up a disproportionate amount of water, leading to increased pressure on the brain tissue in the cranium, which is life threatening.
Respiratory distress can be a complication if underlying pneumonia precipitated the development of ketoacidosis, and may require mechanical ventilation for the patient if there is too much fluid in the lungs (Russell et al. 1981; Reynolds et al. 2002).
Hyperosmolar hyperglycaemic state
A rare event, but more common in people with type 2 diabetes, hyperosmolar hyperglycaemic state (HHS), or hyperosmolar non-ketotic coma (HONK) as it is also known, is produced by a rise in blood sugars, leading to dehydration in the same mechanism as diabetic ketoacidosis, but without acidosis. This is because in the presence of some insulin, there is still the inhibition of glucagon, so only the effects of rising blood glucose levels, and not the complications of fatty acid breakdown, are seen.
HHS normally follows a significant shock to the body such as an infection, cardiovascular event, stroke, or surgery and trauma. As fluid is lost, the relative concentration of blood glucose and red blood cells increases, which causes hypercoagulability and risk of thrombosis. This in turn could cause a stroke or myocardial infarction. Heparin, an anticoagulant, is normally administered to prevent this. Without the slow administration of fluids and insulin, the patient would slip into a coma and die.
Hypoglycaemia
People who are known to have diabetes should be well-versed in checking their blood sugar regularly and eating accordingly in the case of hypoglycaemia. However, when a person with diabetes appears with a particular set of symptoms, it is worth noting that blood glucose should always be checked. These symptoms can roughly be divided into three areas according to their origin:
In low blood sugar, adrenaline is released, causing shakiness, anxiety, palpitations, sweating, pallor (paleness), and pins and needles
Diagnosis of hypoglycaemia is based on Whipple’s triad (Table 4). Other useful factors to take into consideration when diagnosing hypoglycaemia include finding out if the patient is diabetic; finding out a patient’s blood monitor test reading; and seeing if giving them glucose, e.g. in the form of a dextrose tablet, improves their condition. The patient may have administered too much insulin, and glucagon can be injected as a treatment if unconscious.
| 1. Symptoms known to be caused by hypoglycemia. |
| 2. Low glucose at the time the symptoms occur. |
| 3. Reversal or improvement of symptoms or problems when the glucose is restored to normal. |
The obvious pitfall in emergency medicine is to assume that the cause of unconsciousness is diabetic ketoacidosis, instead of hypoglycaemia. If a ‘helpful’ first aider were to use a known person with diabetes’ insulin pen in hypoglycaemia, without checking the blood sugar levels, injection could easily lead to death. This is where being able to recognise the difference between history and signs of diabetic ketoacidosis and hypoglcaemia is hugely important, as well as checking blood sugar levels if at all possible before taking action.
Long-term complications of diabetes
Poor management of diabetes leads to problems primarily based around the hardening of the capillary walls in all areas of the body. This hardening of the capillaries inhibits O2 transfer, leading to cell death.
The nervous system is commonly affected, whereby the cells that put the fatty (myelin) sheaths around neuronal axons are damaged, leading to numbness in the extremities, working their way up the limbs in a ‘gloves and stockings’ distribution. Diabetic ulcers can form as a result of this loss of nerve control to the feet with combined vascular disease. The kidney capillaries harden around the glomerulus, which leads to chronic kidney disease. Retinopathy is also a common complication, with hardening of the capillary walls leading to neovascularisation, which involves the growth of new blood vessels to supply the retina. These new vessels are weak and tend to bleed, obscuring vision.
Unfortunately, as people with type 1 diabetes live with the condition for many years longer due to its predominantly juvenile onset, they suffer more complications of persistently high blood sugar levels. Good consistent management is the key to preventing long-term complications of diabetes, but this is easier said than done, especially in adolescents and young adults.
