In cardiac arrest, the heart will cease from beating suddenly. Cardiopulmonary resuscitation (CPR) provides a manual pressing of the chest to mimic the physical contraction of the heart, thus circulating blood to the vital organs when the heart is unable to beat itself. Poole et al (2018) supports the need for high-quality chest compressions, noting them as a critical component in the chain of survival. As CPR improves survival chances, devices have been developed to support, and replace, manual chest compressions, which are commonly delivered to an inferior quality in clinical practice (Abella et al, 2005; Perkins et al, 2015; Hardig et al, 2017). The concept of the device is to ensure systematic pressing of the chest and therefore prolonged and effective compressions. This, in theory, should allow for human fatigue issues, deliver a consistent pressure and focused timing in line with changing guidelines.
Some studies have suggested mechanical devices can be operator-friendly and easy to use with the improved chance of restarting the heart; aiding survival chances for the cardiac arrest patient (Poole et al, 2018). Furthermore, the mechanical devices allow for manipulation in line with changing guidelines and environments, thus applying the consistency of chest compressions without any impacting human error. The importance of CPR in resuscitating patients and the developments in mechanical chest compression devices have led to this review of such devices compared with traditional techniques.
Aims and objectives
This systematic review will endeavour to demonstrate whether the use of a mechanical chest compression device will be a positive factor in improving survival in the cardiac arrest scenario. In addition, its wider-spread use, association to appropriate environments and patient presentations, linked to safe application will enhance return of spontaneous circulation (ROSC) rates and, ultimately, survival.
Methods
Design
The study question uses the acronym PICO (Population, Intervention, Comparative intervention, Outcomes; Table 1) and forms the systematic review of the current published evidence relating to the deployment of mechanical CPR devices in cardiac arrest, aiming to identify positive and negative aspects of their use in practice.
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| 1. Cardiac arrest | 2. Mechanical chest compressions | 11. Manual chest compressions | 12. Survival to hospital discharge |
| 3. Mechanical CPR | 13. ROSC | ||
| 4. Mechanical CPR | 14. ROSC | ||
| 5. Automated chest compressions | 15.ROSC | ||
| 6. Automated CPR | |||
| 7. Automated CPR | |||
| 8. Powered chest compressions | |||
| 9. Powered CPR | |||
| 10. Powered CPR |
Inclusion and exclusion criteria
All of the studies included were randomised controlled trials (RCTs), helping to ensure that only the highest-quality data were selected (Torgerson, 2003; Bettany-Saltikov, 2012). Consideration for inclusion from the population was patients over 18 years suffering out-of-hospital cardiac arrest (OHCA) or in-hospital cardiac arrest (IHCA), with trained staff completing the CPR. Exclusions were trauma, drowning, hypothermia and toxic substances, due to a differing underlying pathophysiology, requiring a different application of the resuscitation guidance. This will aid generalisation to the population in cardiac arrest. In addition, the intervention delivered was deemed as any device delivering mechanical chest compressions versus human delivery of chest compressions. Subsequently, outcome was measured as any patient with an ROSC or survival-to-hospital discharge.
Data collection
A total of 69 shortlisted papers, as summarised in the PRISMA flowchart (Figure 1; Liberati et al, 2009), were screened by the authors independently to determine whether they met the review criteria. Consequently, each research paper was screened by title, abstract and then the entire article if required, which followed a grading approach. Initially, each study was reviewed and deemed to be either, included or excluded, on the basis of the title's relevance and the research question. An independent reviewer followed the same process to increase the overall validity of the review (Neale, 2009). Snowballing and grey literature were considered but omitted due to tight time constraints and limited resources.
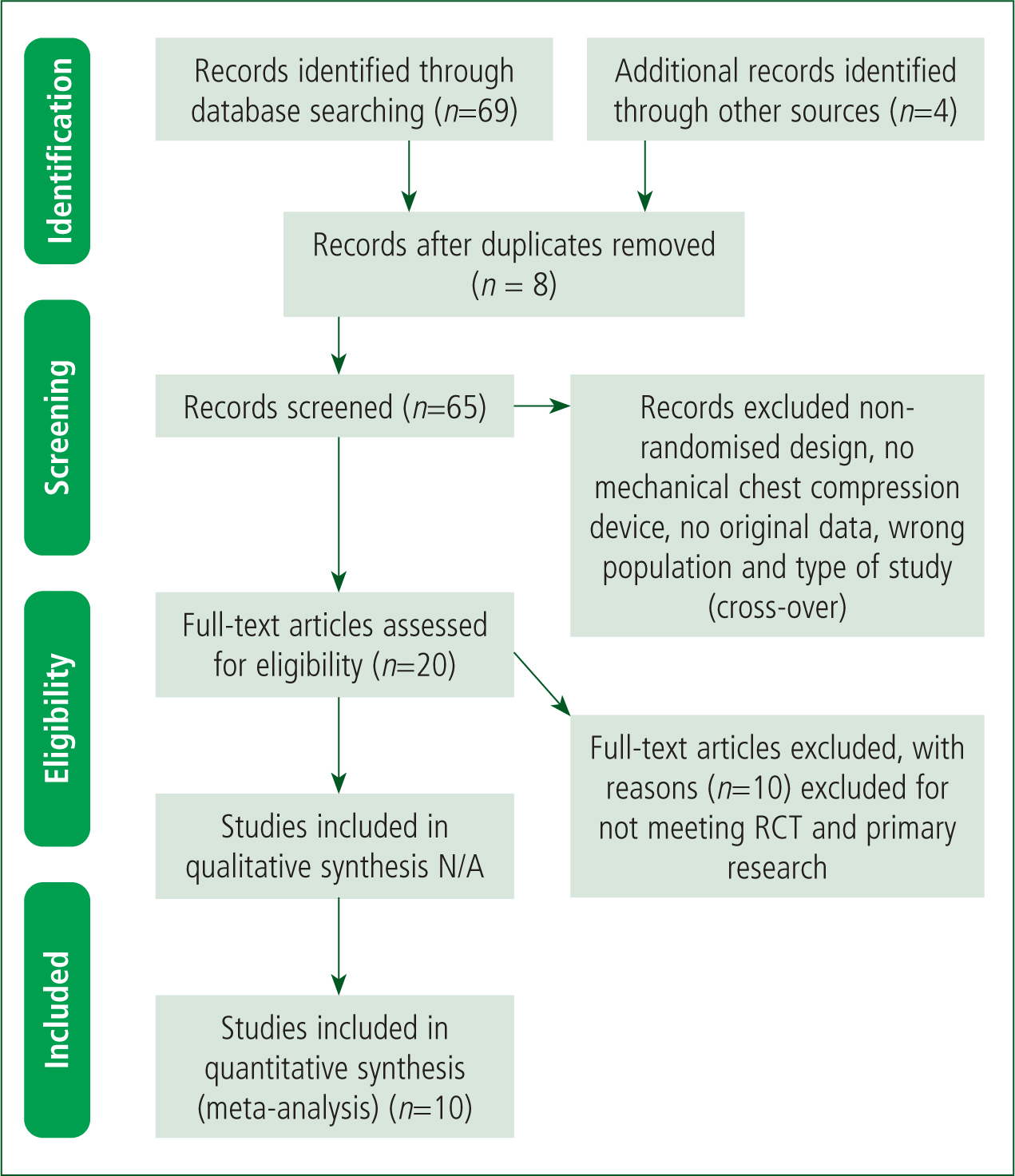
The second, and last stage, was the final selection of papers from the 20 selected from stage 1. Bettany-Saltikov (2012) describes this as reading the full text of the study. There were 10 papers selected for the systematic review and therefore the other 10 papers were excluded because they either didn't meet the inclusion criteria or were not RCTs. The authors chose the Risk of Bias 2 tool, (RoB 2) as this is currently recommended by the Cochrane collaboration and can be easily applied to the RCTs selected. The TIDieR checklist was used alongside the RoB 2 tool (von Elm et al, 2007; Glasziou et al, 2008). This reduced the risk of bias and increased internal and external validity.
Data analysis
A data-extracted form was used to overview the methods, participants, interventions, comparisons, outcomes and notable differentials in guidelines or aspects unique to a study. In addition, a meta-analysis pooled the 10 studies and combined both IHCA and OHCA against the single outcome of ROSC.
Results
The TIDieR checklist summarised the final 10 papers included which were selected from a total of 12 894 patients with three of the studies accounting for 90% of all participants included in the review (Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015). Moreover, several of the studies reported incomplete characteristics for the patients, with a variance in mean age ranging from 45 to 71 years old and subsequent range for male patients was 58% to 68%. There was a significant difference in shockable rhythms (ventricular fibrillation (VF)/ventricular tachycardia (VT)) which varied from 13% to 47%.
There were two studies which were conducted in the USA (Halperin et al, 1993; Dickinson et al, 1998), two in China (Lu et al, 2010; Gao et al, 2016), another from Sweden (Smerkal et al, 2011), one from Holland (Koster et al, 2017) and one from within the UK (Perkins et al, 2015). The remainder of the studies were collaborations between the USA and Canada (Hallstrom et al, 2006), USA/Austria/Holland (Wik et al, 2014) and Sweden/Holland/UK (Rubertsson et al, 2014). None of the aforementioned papers reported on the ethnicity of the patients.
A mechanical chest compression device was used and studied in three small RCTs, in relation to IHCA; sample sizes ranged from 34 to 218 patients with an overall participation of 402 patients across the three studies (Halperin et al, 1993; Lu et al, 2010; Koster et al, 2017). There is evident heterogeneity within the methodology, intervention guidelines application and subsequent reporting in results. Moreover, different mechanical devices were used to deliver chest compressions; a Thumper device, (Lu et al, 2010), a pneumatic device, (Halperin et al, 1993) and the LUCAS and AutoPulse devices (Koster et al, 2017). Furthermore, one study focused on mean aortic pressure (Halperin et al, 1993), another focused on reporting life-threatening resuscitation injuries (Koster et al, 2017) and the latter reporting unclear outcomes in relation to ROSC or survival rates.
OHCA was studied by eight of the RCTs and included 12 492 patients (Dickinson et al, 1998; Hallstrom et al, 2006; Smekal et al, 2011; Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015; Gao et al, 2016; Koster et al, 2017). In addition, Dickinson (1998) evaluated the thumper device in a quasi-randomised study; four further RCTs evaluated the AutoPulse device (Hallstrom et al, 2006; Wik et al, 2014; Gao et al, 2016; Koster et al, 2017) and three RCTs, Koster et al (2017), Rubertsson et al (2014) and Smekal et al (2011), evaluated the LUCAS device. One trial by Perkins et al (2015) evaluated the LUCAS using a cluster-randomised study design.
Results of the studies included are potentially at risk of clinical diversity, also known as clinical heterogeneity. Higgins and Green (2011) suggest that clinical diversity may be present in any systematic review with any potential diversity termed heterogeneity. Any subsequent heterogeneity will be present if there is clinical variation; thus, the true intervention effect may differ across studies. The author notes the potential for clinical diversity including timing of device use, differing types of mechanical device and year of publication (1993–2017), with the latter point having the biggest potential impact. The publication year is significant as guidelines for resuscitation, and manual chest compressions have changed substantially over the past 25 years (European Resuscitation Guidelines, 2015). However, the author conducted a meta-analysis which compared all studies included against the intervention and an outcome of ROSC (Table 2). The pooled odds ratio (OR) of 1.182 (OR 1.182 [95% CI 1.002-1.394]; P<0.047) suggests that mechanical chest compressions are statistically superior. Moreover, the odds ratio reflects a minimal chance of increased survival (ROSC) in the mechanical chest compression control group, with a significant P value (P<0.047). A recent study by Seewald et al (2019) suggests improved outcome for ROSC with mechanical chest compressions appearing superior when risk factors are adjusted.
All of the studies included reported differing outcomes; survival-to-hospital discharge with good neurological function, survival-to-hospital discharge, ROSC, survival to specifics (including rhythm and bystander CPR), adverse effects and time interval delays in certain specifics. Hospital discharge with good neurological function, reported by Hallstrom et al (2006), Rubertsson et al (2014) and Wik et al (2014) demonstrated no significant decrease in survival, although Hallstrom et al (2006) suggested a reduction in survival when mechanical versus manual chest compressions were used. The addition of Rubertsson et al (2014) and Wik et al (2014) again showed differences relating to hospital discharge with good neurological function.
Survival to discharge in several studies focused on potential harm due to the intervention of a mechanical device and showed no significant difference in harm or benefit (Hallstrom et al, 2006; Smekal et al, 2011; Rubertsson et al, 2014; Wik et al, 2014). In addition, two of the studies, Lu et al (2010) and Gao et al (2016), suggested benefit when mechanical chest compressions were employed (Lu et al, 2010: 32.9% v 14.7% RR 2.21, 95% CI 1.18.to 4.17; Gao et al, 2016: 18.8% v 6.3% RR 3.01, 95% CI 1.04 to 8.77).
ROSC was reported in eight of the studies (Halperin et al, 1993; Dickinson et al, 1998; Lu et al, 2010; Smekal et al, 2011; Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015; Gao et al, 2016). Subsequently, four studies minimally favoured the mechanical arm, although these results were not statistically significant (Halperin et al, 1993: OR 4.15, 95% CI 0.86 to 19.92; Perkins et al, 2015: OR 1.01, 95% CI 0.89 to 1.15; Rubertsson et al, 2014: OR 1.04, 95% CI 0.88 to 1.22; Smekal et al, 2014: OR 1.45, 95% CI 0.74 to 2.85). Moreover, three studies established mechanical chest compression benefit (Dickinson et al, 1998: OR 4.85, 95% CI 0.17 to 137.68; Lu et al, 2010: OR 2.03, 95% CI 1.06 to 3.90; Gao et al, 2016: OR 2.67, 95% CI 1.26 to 5.63). The final study by Wik et al (2014) favoured the manual arm (OR 0.84, 95% CI 0.74 to 0.96).
Data relating to timing of certain interventions was recorded, and reported, by four studies (Hallstrom et al, 2006; Smekal et al, 2011; Rubertsson et al, 2014; Wik et al, 2014). The Wik et al (2014) trial demonstrated a marginal delay in defibrillation time in the mechanical arm. Hallstrom et al (2006) reported call to first shock interval in the mechanical arm, although the call to rhythm check data were similar (call to first shock interval 11.8 ± 6.1 minutes versus 9.7 ± 3.1 minutes, P - 0.001). Moreover, Rubertsson et al (2014) reported data which included a 1.5-minute delay in defibrillation within the mechanical arm. This can be partly attributed to the protocol which prescribed a first defibrillation after 90 seconds, without a rhythm analysis. Smekal et al (2011) contrasts these three studies with data which suggest an average scene time to CPR, device application and commencement of CPR and call to start of CPR.
Attempting any correlation between initial rhythm and outcome was only reported by two studies (Hallstrom et al, 2006; Perkins et al, 2015). This suggested inadequate data and a greater risk of heterogeneity to attempt any pooling of data. Perkins et al (2015) reported a lower survival rate in the mechanical arm, linked to a rhythm which required defibrillation (OR 0.71, 95% CI 0.52 - 0.98). VF/VT). Their non-shockable patients showed no difference (OR 1.38, 95% CI 0.80 - 2.36). Furthermore, Hallstrom et al (2006) stated a higher 4-hour survival rate for the mechanical arm (17.2% v 10.4%). However, this was not reported with any statistical data, noted simply as a potential ‘trend’ by the study authors.
Discussion
Results revealed significant heterogeneity in relation to patients selected (both OHCA and IHCA), timing of device application, type of mechanical device used, CPR protocol guideline employed in the control arm and underlying cardiac arrest cause.
Patients who survived OHCA to discharge with an intact neurological function was reported by three studies (Hallstrom et al, 2006; Rubertsson et al, 2014; Wik et al, 2014). Subsequently, two trials, Rubertsson et al (2014) and Wik at al (2014), reported no significant difference in survival to discharge with intact neurological function. Wik et al (2014) determined a similarity between manual and mechanical arms in relation to the same outcome. In addition, three trials that reported short-term survival, less than 30 days, produced no data to suggest any difference in survival in either arm (Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015). However, Perkins et al (2015) did report favourable neurological function after 3 months (OR adjusted), which suggests manual chest compression superiority to 30-day survival. A smaller study by Dickinson et al (1998) reported an ROSC in 10% of the patients, although the total sample was only 20 patients.
Larger trials reported no significant evidence in longer-term outcome (survival), with no significant adverse effects in either control arm or a robust methodology for compression-related injuries. Previous data suggest that mechanical devices provide a consistent rate, depth of compression and haemodynamic stability, compared with manual, although this appears to conflict with the evidence in the present study and a lack of improvement in ROSC with a mechanical device. In addition, device deployment may impact on the acute phase where haemodynamic stability is at its most significant, suggesting mechanical device limitation.
The quality of the actual manual chest compressions is highly dependent on the compressions delivered within the manual arm. Focusing on control groups, an inferior quality manual chest compression will support the mechanical device. Wik et al (2014) reported a 79% compression time in both arms, CPR and training, with a decrease in manual CPR. Rubertsson et al (2014) also reported 78% in their manual CPR; however, Wik et al (2005) allude to standard 50% compression timing in normal practice. Consequently, deploying mechanical devices in practice may differ from manual compression quality established by the trials within this review, which note high-quality chest compressions within the control group.
The majority of the included studies focused on two devices, the AutoPulse and the LUCAS, which focused on OHCA in the vast majority of patients, with large sample sizes. As the main focus of the current review was survival rates (ROSC) using a mechanical device, the decision was made to exclude any studies with non-randomised selection of patients. Moreover, the rationale included potential bias, differentials in care quality within healthcare systems and historical practice changes, leading to the potential for the Hawthorne effect as allowing studies to select participants, focusing on IHCA settings and changes in CPR guidelines driven through the evidence base support this effect. Furthermore, selection bias may be prevalent, where patients are selected in the hope of improved outcome, although they may have a limited chance of survival (Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015).
The defibrillation effect on mechanical device performance must be considered. Two studies include outcome data relating to initial rhythm, with improved outcome in a non-shockable presentation. (Hallstrom et al, 2006; Perkins et al, 2015). With no reported data for treatment or time pauses, the effectiveness of the mechanical device may be affected by deployment timing and defibrillation. Ultimately, these data may prove significant in performance of the mechanical device.
All of the studies included have limitations which relate to concealment of allocation and randomisation (risk of bias). Therefore, selective reporting bias was unclear, although guideline protocols for both manual and mechanical CPR are reported in the more recent studies (Rubertsson et al, 2014; Wik et al, 2014; Perkins et al, 2015). Moreover, the older studies lacked clarity and description of protocol and CPR quality, which is deemed a significant methodological limitation and risk of bias, with variation in CPR application across studies. Moreover, Rubertsson et al (2014), as an example, used a specific protocol for the mechanical arm within their study, where defibrillation was instigated without a rhythm analysis. This suggests co-intervention issues, where treatment does not align with resuscitation guidelines, within the manual arm, thus affecting survival outcome and ROSC. The study also followed the 2005 resuscitation guidelines and is therefore outdated. Further to this, it is challenging to differentiate between the observed treatment effect for CPR application and mechanical device. In addition, a further study used a modified CPR protocol in its mechanical arm, again suggesting potential co-intervention (Smerkal et al, 2011).
Perkins et al (2015) employed training in a pragmatic manner using locally agreed organisational practices, with a focus on the quality and application of CPR through initiating training of operator and monitoring compliance. Consequently, it is challenging to analyse compliance as only 60% of the mechanical arm received a combination of manual and mechanical chest compressions; potential challenges encountered in device application may be reflected as a result. A partiality in application by the operator may be present due to a thought process supporting conventional manual compressions or a lack of confidence with its use. Therefore, data may be interpreted as an undervalued efficacy of true treatment effect of mechanical compressions or provide a true estimation of mechanical compressions provided.
Hallstrom et al (2006) related mechanical chest compressions to inferior outcomes although there were a number of potential risks for bias. Shockable rhythm patients were even in the mechanical arm, although there was an average delayed defibrillation time of over 2 minutes. Moreover, there were three options for application of the device within the protocol and an absence of complete compression observation, thus suggesting a delay in device application and a potential to reduce this time if the operators had improved training in deployment and application. This time delay may be accountable for the differential in manual and mechanical arms. In addition, Paradis et al (2010) noted that a mechanical harm risk was attributed to a single study site with mid-point changes in protocol as a result of this, which encompassed delay in device deployment. In contrast, the Auto Pulse device may have its efficacy impacted by morbidly obese and thinner patients in the mechanical group and unaccounted for in the study. Although this is not specifically reported on in the data, this may impact on the overall results in the study. As outcomes prior to the study are unreported, it is challenging to predict the consequence (positive) of the Hawthorne effect on manual compressions, thus potentially making it difficult for the mechanical device to demonstrate supremacy due to size of the participant. Consequently, the study authors did note the significance of device application and the correlation with outcomes.
Wik et al (2014) focused on developing a design to combat some of the Hallstrom et al (2006) design limitations. The result was a robust measurement of compressions, in three phases, using thoracic impedance, thus reducing the risk of bias. The authors noted an increase in the quality of normal compressions (greater than normal), which they reported as statistically significant and therefore equal to mechanical compressions. Furthermore, several of the studies failed to define patients who were included in the studies in respect of variables, such as speed of compression and gender, which are known to be related to patient survival. Lu et al (2010) did not provide initial rhythm data for patients, which may shed some light on disparity between treatment groups. This could thus be a significant factor involving prognosis and support observational differences in ROSC.
There is noteworthy heterogeneity among the studies included within the present review relating to intervention, patient population and applied protocols. However, the author decided to conduct a meta-analysis to try and add statistical data to the review. Consequently, the 10 studies included were pooled, both IHCA and OHCA, against a single outcome of ROSC. The results produced an odds ratio (OR) of 1.182 (OR 1.182, [95% CI 1.002-1.394]; P<0.047), where the OR reflects a minimal chance on increased survival (ROSC) in the mechanical chest compression control group, with a significant P value, (P<0.047). In addition, one study skewed this result which had a negative impact on the statistical value. Dickinson et al (1998) is the oldest study and had the smallest sample size of 20 patients. The outcome data reports a 10% ROSC with one admission to hospital and no survival to discharge, suggesting a significant risk of negatively impacting the statistical data produced in the meta-analysis.
Conclusion
Overall, the evidence analysed suggests that mechanical chest compression devices are statistically superior to manual chest compressions of a high quality, when up-to-date protocols and guidelines are followed. It can be noted that the evidence demonstrates that mechanical chest compressions are not superior to manual and therefore comparable without the statistical data. Therefore, the author determines that on the balance of probability, and evidence, that mechanical chest compression devices employed by trained health professionals are not only an equitable alternative, but also a practical and beneficial addition to a patient's resuscitation. Future research should focus on compression accuracy, non-shockable rhythm presentations which remove the need for defibrillation, patient size and under 18s.

