Subarachnoid haemorrhage (SAH) carries a substantial burden of morbidity and mortality—therefore, the development of methods to rapidly detect SAH with high sensitivity is very important. Paramedics are frequently called to patients complaining of headache and the treatment for patients with clinically likely SAH is mostly straightforward for prehospital clinicians. However, a number of patients present with atypical symptoms and 12% of all SAHs are overlooked on initial assessment (Kowalski et al, 2004).
The study being discussed in this article aimed to develop a new clinical decision rule using only objectively measurable predictors to exclude SAH. The authors aimed to offer higher specificity than the previously developed Ottawa SAH rule, an evidence-based, two-stage clinical decision rule, while maintaining Ottawa's 100% sensitivity.
A total of 1899 patients aged over 15 years old with a main complaint of headache and presenting within 14 days were considered for enrolment from five general hospitals in Japan between April 2011 and March 2014. Patients were aged 16–98 years of age with a mean age of 53.
The exclusion criteria included:
After application of the exclusion criteria, 1561 patients were eligible for enrolment in the study, of which 277 had an SAH and 1284 did not.
The results of univariate analysis showed that patients with SAH were older; they more frequently showed onset during exertion, complained of the ‘worst headache of my life’; experienced altered consciousness, neck pain or stiffness, and vomiting; and had history of hypertension. By using this analysis of risk factors, the authors devised an ‘Ottawa-like’ rule which used six risk factors to predict SAH.
A second analysis, this time of objectively measurable variants, showed that blood pressure (mean=167/93 mmHg vs. 139/81 mmhg) and for unknown reasons blood glucose level (9.0 mmols/litre vs. 7.0 mmols/litre) were higher in patients with SAH, but body temperature (36.3°C vs. 36.6°C) and serum potassium levels (3.6 mEq/litre vs. 3.9 mEq/litre) were slightly lower. Temperature differences were not included within the rule itself. The objectively measurable variants formed the ‘EMERALD SAH’ rule. By combining the Ottawa SAH rule and their EMERALD SAH rule, the authors proposed a two-step decision-making rule to exclude SAH (Figure 1).
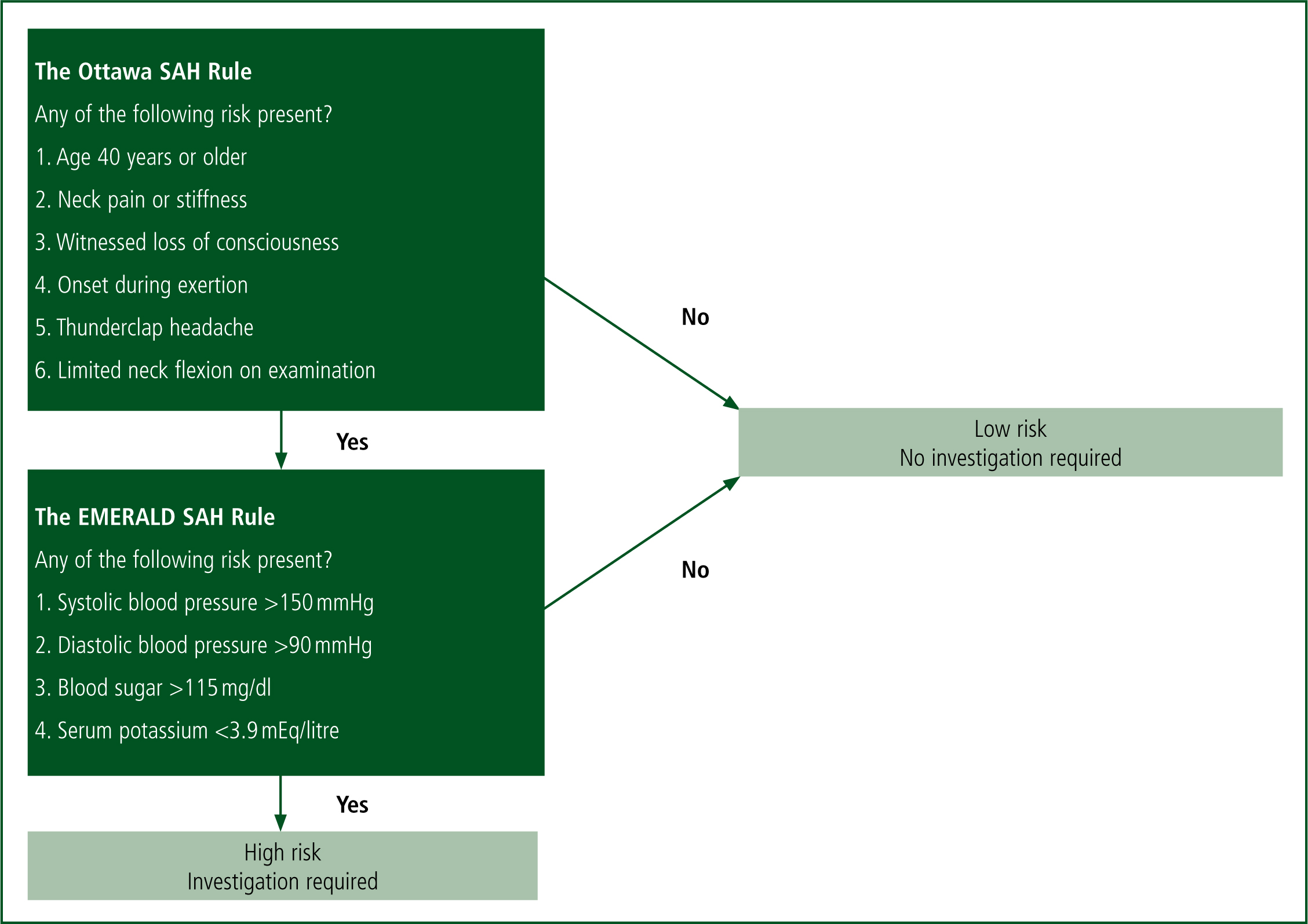
Kimura et al (2016) placed the Ottawa SAH rule as the first screening step because it is more clinically intuitive and blood sampling is not needed. If the patient scores negatively in the Ottawa rule, or positively in the Ottawa then negative in the EMERALD rule, they are deemed low risk and computed tomography (CT) and lumbar puncture are not indicated.
The authors justifiably state that for a disabling and life-threatening condition such as SAH, it is essential that 100% sensitivity is achieved if a clinical decision rule is proposed. This study finds 100% sensitivity in both the Ottawa and EMERALD rules, with a specificity of 8.8% in Ottawa, and 14.5% in EMERALD. The quality of the research is limited by the fact that 188 patients were excluded as they were either lost to follow-up or had no CT images.
The use of such a clinical decision rule can be justified not only to detect SAH, but its implementation could also reduce high costs of CT radiography and lessen pressure on hospitals. It can also reduce the risk of introducing an iatrogenic post-lumbar puncture headache, which the authors note can be worse than the presenting headache itself. The article acknowledges that the proposed rule needs to be accurately validated before being fully incorporated into clinical practice—currently it has only been internally validated. Prehospital validation would also be required prior to prehospital implementation. Additionally, as serum potassium is not routinely measured by ambulance paramedics in the UK, this does provide a further limitation.
However, the Ottawa rule on its own has been validated by Perry et al (2017) and would be likely to be appropriate for prehospital use to rule in SAH. Of course, the Ottawa rule is only intended for identifying patients with SAH and cannot be used to exclude other headache types that require further investigation.
Paramedics are working at the forefront of patient care and the way we start the patient's journey has the potential to influence the patient's future clinical course. Future use of the two-stage Ottawa and EMERALD rule could be used by paramedics as a rapid recognition tool to pathway the patient to a neurosurgical centre and to handover an evidence-based high index of suspicion of SAH to our hospital-based colleagues. Careful application of the EMERALD SAH rule in emergency departments with a low prevalence of SAH would appear to be warranted—however, caution in its use is needed.

