Patients with severe infections and sepsis frequently present to emergency departments, with many of these patients being transported by ambulance services. Septic patients account for 27 % of admissions to intensive care units in England (Robson et al. 2009) and are responsible for 50 % of critical care resources (Daniels et al, 2011). Their average hospital stay is four weeks (Robson et al. 2009) with 37 000 patients dying of sepsis in the UK each year (Daniels et al, 2011).
In spite of clear treatment guidelines produced by the European Society of Critical Care Medicine and the Society of Critical Care Medicine, patients with sepsis still have a mortality six times greater than those presenting with acute myocardial infarction and five times greater than those presenting with acute stroke (Cronshaw et al. 2011).
However, only 18.8 % of UK emergency departments are able to initiate the suggested treatments in the recommended time frame (Sivayoham, 2007), recent initiatives between emergency departments and ambulance services have led to considerable improvements in the care of patients with myocardial infarction, acute stroke and multiple trauma, with the speed of treatment dramatically improving patient outcomes (Dellinger et al, 2004). Paramedics have been central to these improvements, providing early recognition, early interventions, and rapid transportation to appropriate receiving units while providing pre-alerts to enable the mobilisation of specialist teams and further reducing the time to definitive care. Pre-hospital staff could make similar contributions to patients with severe sepsis, and are well placed to promote the delivery of early goal–directed therapy.
The pathophysiology of sepsis
Sepsis is a common condition in which the body over reacts to a severe infection. During a period of infection a normal inflammatory response is essential, as the body increases the permeability of capillaries to allow leukocytes to enter the surrounding tissues and fight the invading pathogen (Tortora, 2008). In healthy individuals, this response is downgraded once the pathogen has been removed, however in sepsis, the balance between activation and down regulation fails, leading to unregulated coagulation, localised ischaemia, shock and organ failure (Porth, 2007). Untreated mortality rates of severely septic patients approach 50 % (Robson, 2004).
Bacteria are a common cause of inflammation, with half of all patients who acquire sepsis doing so from a lower respiratory tract infection (Nee, 2006). Other common sites of infection include abdominal injury, for example from a ruptured diverticulum, and urinary tract infections (Stearns-Kurosawa et al, 2011), however sepsis may also result from trauma, burns or soft tissue injuries. Receptors present on the surface on certain cells recognise these foreign stimuli and release inflammatory mediators, recruiting leukocytes into the infected area and increasing the permeability of blood vessels. This allows plasma and leukocytes, in particular neutrophils, to migrate into the surrounding tissues where they begin the phagocytosis of pathogens (Porth, 2007). This increase in local extravascular fluid and the dilation of capillaries can be seen in the characteristic heat, redness and swelling of an inflammatory response (Tortora, 2008)
‘…an unregulated release of inflammatory mediators are released leading to a vastly exaggerated immune response…’
The process of inflammation requires continual stimulation, with negative feedback mechanisms ensuring the response is reduced once the stimulus is removed. In most situations a short period of localised inflammation is sufficient, however in sepsis, an unregulated release of inflammatory mediators are released leading to a vastly exaggerated immune response (Porth, 2007). As sepsis progresses, widespread inflammation leads to alterations in the coagulation system, allowing blood to clot when it would normally flow well within vessels (Butler, 2008). This leads to the formation of microthrombi, reduced blood flow, poor tissue perfusion, reduced oxygen delivery and organ dysfunction (Dolan, 2003). Meanwhile, metabolic rate and oxygen demand are increased as a result of the infection, with the subsequent mismatch between demand and availability further increasing tissue hypoxia (Rivers et al, 2001). This increase in tissue hypoxia also leads to the disruption of coagulation haemostasis, and a continuing progression of circulatory failure (Rivers et al, 2001). Eventually, localised inflammation becomes overwhelming, leading to a systemic reaction, multiple organ damage and haemodynamic compromise (Dolan, 2003).
The inflammatory response may be classified into one of four categories: 1) a systemic inflammatory response (SIRS), 2) sepsis, 3) severe sepsis, or 4) septic shock (Table 1). SIRS is diagnosed when a patient has two or more of: 1) a temperature greater than 38 °C or less than 36 °C, 2) a heart rate greater than 90/min, 3) a respiratory rate over 20/m, or 4) a white blood count under 4000 mm3 or over 12000 mm3 (Chege and Cronin, 2007). Sepsis is defined as SIRS in the presence of a known or suspected infection (Remick, 2007) while severe sepsis is sepsis accompanied by organ dysfunction, hypoperfusion or hypotension (Nee, 2006). Septic shock is diagnosed when patients with severe sepsis have hypotension which is unresponsive to fluid challenges (Nee, 2006).
| SIRS |
|
| Sepsis | SIRS plus known or suspected infection |
| Severe sepsis | Sepsis plus organ dysfunction, hypoperfusion or hypotension |
| Septic shock | Severe sepsis plus hypotension unresponsive to fluid |
Surviving sepsis campaign
Sepsis is clearly a complex condition with a high mortality, and therefore presents a significant challenge to emergency care teams. In recognition of its severity, 2002 saw the European Society of Critical Care Medicine and Society of Critical Care Medicine provide a consensus view towards an agreed definition and treatment plan (Surviving Sepsis Campaign, 2011). The response was an agreement on the management and recognition of sepsis, with an emphasis on the impact of early goal directed therapy (EGDT) and treatment ‘bundles’ aimed at improving survival rates (Dellinger et al, 2004). Launched in 2002, with further updates in 2004 and 2008, the surviving sepsis campaign aims to increase knowledge and awareness of the condition, define care standards and reduce mortality by 25 % (Robson et al. 2009)
Although written primarily for intensive care units (ICUs), the campaign found that treatment should not be delayed until ICU admission and should instead begin the moment sepsis is recognised (Dellinger et al, 2004). This resulted in two treatment paths, the ‘sepsis six’ bundle, which should be delivered within the first six hours following recognition of severe sepsis, and the ‘management’ bundle which should be completed within the first 24-hours. The sepsis six bundle and its emphasis on early recognition, management and treatment is particularly applicable to emergency and pre-hospital care.
The sepsis six (Table 2) recommends that in the first six hours following diagnosis, patients should receive high flow oxygen, have blood cultures taken prior to antibiotics, be administered intravenous antibiotics, have their serum lactate measured, be given fluid resuscitation and have their urine output measured (Daniels et al, 2011).
The management bundle recommends vasopressors for persistent septic shock (Dellinger et al, 2008), low dose steroids for septic shock, maintaining glucose control at > 8.3 mmol/l and carefully selected tidal volumes for patients requiring mechanical ventilation (Surviving Sepsis Campaign, 2011). These recommendations have been shown to significantly improve survival in patients with severe sepsis, demonstrating a 34.4 % reduction in mortality (Daniels et al, 2011). In one prospective observational study of 567 patients presenting to an acute UK hospital, only 5.9 % of those receiving the full resuscitation bundle died compared to 51 % of those who did not (Daniels et al, 2011).
Treating the septic patient
The focus of treatment for the septic patient is on removing the infection with appropriate antibiotics while providing supportive treatment to maintain circulation (Porth, 2007). Treating the illness while confirming the diagnosis is recommended, with a 7.6 % increase in mortality for each hour antibiotic treatment is delayed (Cronshaw et al. 2011). Other interventions, such as fluid and oxygen therapy, are aimed at preventing deterioration and avoiding organ failure (Rivers et al, 2001).
Fluid resuscitation is the first priority for patients with severe sepsis or septic shock (Dellinger et al, 2004) with aggressive fluid replacement compensating for the hypoperfusion and third spacing caused by vasodilatation (Porth, 2007). Intravenous fluids aim to increase circulating volume in order to maintain perfusion pressure and avoid organ dysfunction (Chege and Cronin, 2007).
A challenge of between 500 ml and 1000 ml of crystalloids over the first 30 minutes is recommended (Dellinger et al, 2008) with further research suggesting a bolus of 20 ml/kg (Chege and Cronin, 2007). Robson et al (2009) suggest a total fluid challenge of up to three boluses, effectively providing the hypotensive septic patient with 60 ml/kg. Hypotension which remains unresponsive to fluid challenges is likely to be treated with vassopressors, including norepinephrine, dopamine or dobutamine (Dellinger et al, 2008).
Supplemental oxygen treatment is also important, as septic patients have increased oxygen demands due to the increase in metabolism together with hypoperfusion and localised ischaemia from increased coagulation (Remick, 2007). Over half of all septic patients acquire the illness from a lower respiratory tract infection, such as pneumonia or acute bronchitis (Nee, 2006), and as such, are likely to have compromised respiration before the illness progresses. These factors lead to an overall reduction in tissue oxygenation, with severely septic patients having a significantly increased oxygen demand together with reduced delivery (Robson, 2009).
Ambulance service clinical guidelines recommend high flow oxygen for all unstable patients ( Joint Royal Colleges Ambulance Liaison Committee (JRCALC), 2006), and these patients will certainly require supportive oxygenation. In hospital, treatment involves the measurement of central venous oxygenation, with a target saturation of 75 % (Chege and Cronin, 2007), however pre-hospital treatment should be measured by pulse oximetry with a target saturation of at least 94 % (Robson et al. 2009).
Although fluid resuscitation and supportive oxygen therapy aim to prevent further deterioration, removing the infection remains the priority of treatment. With the prognosis worsening for each hour in which antibiotic treatment is delayed, broad spectrum antibiotics should be given within the first hour following diagnosis while blood cultures can ensure a more appropriate prescription within 48–72 hours (Nee, 2006). Choice of appropriate antibiotics is dependent on many factors, such the clinical history, the allergies of the patient, the clinical presentation, recent antibiotic use and local resistance patterns (Dellinger et al, 2008), with a broad spectrum agent (such as piperacillin with tazobactam) recommended for community acquired sepsis ( Joint Formulary Committee, 2012).
Implementation
Despite clear guidelines on the recognition and management of sepsis, as well as research demonstrating the improvement in outcomes delivered by EGDT, implementation of the sepsis guidelines remains poor. Effective treatment is time sensitive with emergency departments playing a key role in recognition and implementation. However, in a study of 107 UK emergency departments, Sivayoham (2007) found that only 18 % were able to initiate the pathway towards EGDT, with just 55 departments having a written protocol for treating sepsis and only 35 of those including EGDT. Of the emergency departments questioned, only 71 % had heard of the surviving sepsis campaign (Sivayoham, 2007).
In a study of 2461 emergency clinicians across the UK, USA and Australia, Reade (2010) found that just two respondents would comply with all elements of the surviving sepsis campaign when presented with a hypothetical scenario, with less than a third giving the recommended amount of fluid (Reade, 2010). In a study of 255 patients admitted to three hospitals with severe sepsis or septic shock, Cronshaw et al (2011) found that only 17 % were given a diagnosis of sepsis by emergency department staff and only 41 % of patients given a diagnosis of severe sepsis were given the recommended care standards.
‘…with septic shock more likely to be avoided if appropriate treatment is started early many elements of the sepsis six bundle could be commenced en-route to hospital…’
Explanations for poor implementation may include the difficulty in recognising septic patients in busy emergency departments, the complexity of EGDT treatment, doubts over the validity of the original research and staff resources (Reade, 2010). Many healthcare staff receive little training in its identification, and the importance of early and aggressive treatment is often not realised (Chege and Cronin, 2007). Delays may also result from differential diagnoses, for example acute heart failure, poor communication with ICU colleagues and delays in triage (Cronshaw et al. 2011).
Role of paramedics
Patients with severe sepsis are six times more likely to die than patients with acute myocardial infarction and have a mortality five times greater than those with acute stroke (Cronshaw et al. 2011). Early recognition and treatment of these patients by paramedics has led to significant improvements in care, with paramedic activation of cardiac catheter labs leading to significant reductions in onset to reperfusion times. In one study of 841 patients presenting to a UK hospital, a reduction in call to angioplasty of 102 minutes to 39 minutes was demonstrated with paramedic–led recognition of myocardial infarction and activation of cardiac teams (Kunadian et al, 2010). Similar pathways have been recommended for stroke patients, with pre-hospital recognition and pre-alerting aiming to reduce the time from onset of symptoms to a computerised tomography (CT) scan and subsequent reperfusion. Paramedics play key roles in the recognition of these patients and an opportunity exists for pre-hospital staff to make similar improvements in the care of patients with severe sepsis.
Although guidelines were initially directed towards ICU settings, their implementation in emergency departments is vital if the resuscitation bundle is to be provided. Similarly, these guidelines should be implemented and encouraged in the pre-hospital environment to further reduce delays to definitive treatment. Significant numbers of patients with severe sepsis are admitted to hospitals through emergency departments (Robson et al. 2009) with 60 % of patients in the surviving sepsis database coming from emergency department admissions (Butler, 2008). It is likely that many of these patients are transported by ambulance services, and with septic shock more likely to be avoided if appropriate treatment is started early many elements of the sepsis six bundle could be commenced en-route to hospital (Robson et al. 2009).
The key treatments in the early stages of severe sepsis include the maintenance of circulation and oxygenation, both of which are achievable prehospitally. Paramedics can provide early fluid resuscitation and should have confidence in giving aggressive fluid challenges. With many clinicians giving less fluid than is recommended for hypotension (Reade, 2010) and with the surviving sepsis campaign recommending high volumes of fluid in the initial stages of treatment (Dellinger et al, 2008), paramedics should calculate doses of between 12 ml/kg and 20 ml/kg of crystalloid and be prepared to repeat such doses up to three times (Robson et al. 2009). It should be noted that the crystalloid fluids used in pre-hospital care may lead to oedema in greater volumes when compared to colloid solutions, and care should therefore be taken to avoid pulmonary overload. High flow oxygen should also be used in line with ambulance clinical guidelines as these patients have increased demand yet reduced delivery. Although the surviving sepsis campaign guidance recommends monitoring central oxygen saturations, this is not achievable pre-hospitally, and paramedics should use instead use pulse oximetry and provide oxygen via anon-rebreather mask to maintain saturations of 94–98% (JRCALC, 2006).
Other elements of the sepsis six include measuring serum lactate, measuring urine output, taking blood cultures and administering broad spectrum antibiotics. Lactate accumulates as a result of anaerobic respiration and tissue hypoxia, with increased levels a reliable indication of shock and organ dysfunction (Robson et al. 2009). Patients with severe sepsis and septic shock have high levels of serum lactate, with higher levels indicating increased severity of the illness (Robson et al. 2009). A lactate higher than 4 mmol/l, together with a diagnosis of SIRS greatly increases the possibility of ICU admission, with an elevated measurement for more than 24 hours being associated with mortality rates as high as 89 % (Bryant et al, 2004). It is therefore a useful signal of tissue hypoxia and organ dysfunction, even in the absence of hypotension, with McClelland et al (2012) recognising its diagnostic value over other observations such as pulse and blood pressure. Daniels et al (2011) recommend fluid resuscitation for patients with raised lactate levels even in the absence of reduced blood pressure with Robson et al (2009) noting that pre-hospital lactate measurement would improve the treatment of severely septic patients who do not demonstrate any other signs of organ dysfunction.
The value of measuring serum lactate is clear, and its addition to the diagnostic capabilities or paramedics would provide an important tool to improve the treatment of septic patients. Perhaps most importantly, measuring serum lactate prehospitally would allow paramedics to recognise the severity of the illness and to commence fluid resuscitation in severely ill patients who are maintaining a normal blood pressure. Portable lactate monitors are commercially available and similar in design to blood glucose monitors, however the current cost of approximately £400 (HAB Direct, 2012) may prevent their addition by ambulance trusts. Further training would also be required in order for paramedics to understand the significance of findings.
Taking blood cultures and providing antibiotics pre-hospitally could also aid in the treatment of patients with sepsis. Between 30 and 40 % of patients with a clinical presentation of sepsis have a positive culture (Chege and Cronin, 2007) with taking cultures before providing antibiotic treatment giving the best chance at identifying the causative agent and providing the appropriate antimicrobial treatment (Dellinger, 2008). Patients with severe sepsis and septic shock will be given intravenous fluids by paramedics, so taking additional blood while gaining intravenous access is unlikely to result in significant delays in transport. Difficulties may arise from different receiving units having different policies and procedures, while cross contamination from difficult pre-hospital environments could prove a barrier to the reliability of samples. It is likely that such agreements would need to be agreed locally between ambulance trusts and receiving hospitals, with differing transport times between rural and urban services perhaps being a factor. Rural services with prolonged transport times may find pre-hospital interventions more beneficial compared to inner city trusts.
‘…most importantly, measuring serum lactate pre-hospitally would allow paramedics to recognise the severity of the illness…’
Pre-hospital antibiotic treatment by paramedics would further improve the treatment of patients with severe sepsis. Mortality of these patients increases by 7.6 % for each hour antibiotic treatment is delayed (Cronshaw et al. 2011), and with the surviving sepsis campaign recommending antibiotic treatment within an hour of diagnosis (Dellinger et al, 2008), early treatment by paramedics would improve both compliance with this recommendation and survival rates. Sterilisation of blood cultures occurs within hours of antibiotic administration (Robson et al. 2009), so it is likely that giving pre-hospital antibiotics would be dependent on the feasibility of taking pre-hospital blood cultures; providing pre-hospital antibiotics while not taking pre-hospital blood cultures could prevent accurate identification of the causative agent and subsequent targeting of appropriate therapy once in hospital. Importantly, it should be noted that paramedics are already licensed to provide benzylpenicillin to patients with meningitis under patient group directives, local policies may therefore allow further broad spectrum antibiotics to be available to paramedics for treatment of septic patients.
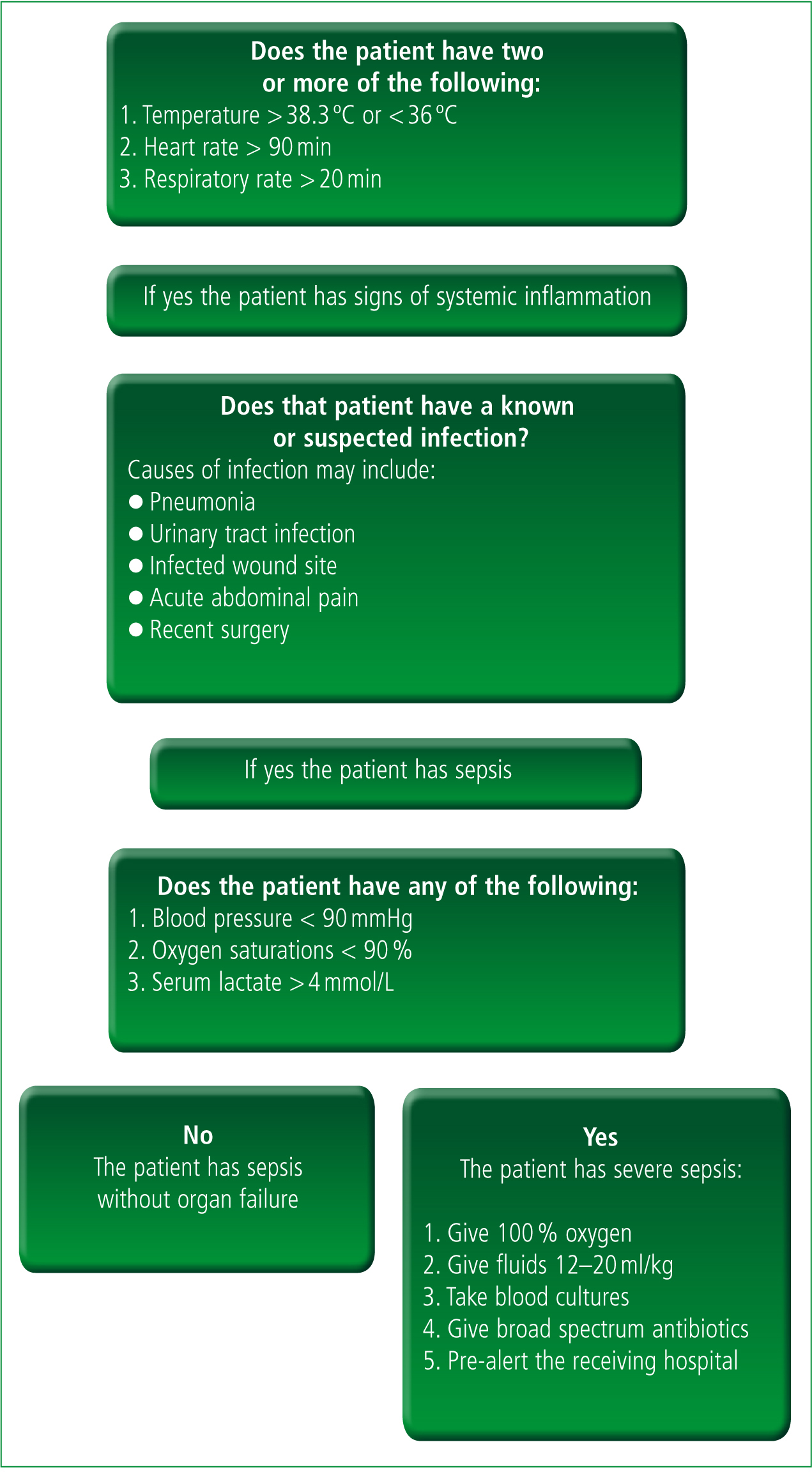
Perhaps more important than providing clinical interventions, paramedics can improve their recognition of severe sepsis and accept that delays in the care of these patients are unacceptable (Robson et al. 2009). As well as improving recognition, rapid transportation is also important as is pre-alerting receiving units to ensure that critical care teams are standing by to continue early treatment. Despite ambulance clinical guidelines stating that pre-alerting hospitals with time critical patients is essential (JRCALC, 2006) one small study of patients admitted by ambulance to an acute UK hospital (n = 454) found that 56 % of critically ill or injured patients arrived at the emergency department with no prior warning (Brown and Bleetman, 2006) with medically unwell patients at particular risk of being under alerted. Paramedics need to ensure that they are able to identify critically unwell septic patients and that they provide hospitals with alerts to ensure the mobilisation of appropriate teams. A simplified screening tool, such as that adapted from Robson et al (2009) in Figure 1 may aid decision making and treatment.
Conclusions
Severe sepsis is a complex illness with a high mortality in the absence of early aggressive treatment. Early goal directed therapy is shown to significantly reduce mortality, yet implementation of the recommended treatment bundles remains poor. Paramedics have demonstrated how they can improve the care of patients with myocardial infarction and stroke through early recognition, treatment and rapid transportation, and are well placed to provide similar improvements to the care of patients with severe sepsis. The use of a pre-hospital screening tool may help paramedics to identify these patients, to provide early treatment and to implement the mobilisation of specialist teams to continue care. Further discussions regarding pre-hospital lactate measurement, taking of blood cultures and providing broad spectrum antibiotics could encourage further reductions in the mortality of these patients.
