It is estimated that there are around 3 500 confirmed cases of bacterial meningitis and septicaemia in the UK per annum (Joint Royal Colleges Ambulance Liaison Committee (JRCALC) and Meningitis Research Foundation (MRF), 2008). Meningococcal diseases can have profound long-term debilitating effects and high mortality rates, especially in young children and adolescents; therefore, it is vitally important that menigococcal diseases are recognised as early as possible, with rapid intervention. In recent years, there have been a number of widespread media campaigns emphasising the early recognition of meningococcal disease, together with published guidelines for the management of the disease in both pre-hospital and hospital settings.
This article will focus on the pathophsyiology and aetiology of the identified disease process, potential complications, together with the pre-hospital and hospital-based management. A number of sources of evidence have been identified, which relate to the pathological processes occurring in disease, some of which are more dated than others. However, despite this, these sources provide relevant theory that is widely acknowledged and accepted, and has seen little change throughout recent years.
Pathophysiology and aetiology
A large variety of organisms, such as meningococci, pneumococci and influenza type b, normally inhabit the naso- and oropharnyx, causing relatively no harm in a healthy individual. Predisposing infections cause a weakening in the immune system, enabling organisms to cross mucosal membranes and invade the body, thus causing bacterial, viral or fungal infections, with bacterial being the most life threatening (Boss, 2006; Peate, 2011). When meningococci organisms cross these mucosal membranes, they rapidly multiply and are transported around the body within the bloodstream. Within some individuals, the bacteria cross the blood-brain barrier, enabling bacteria to enter the central nervous system, colonising and multiplying rapidly within the cerebral spinal fluid (CSF) and meninges (Boss, 2006). This causes irritation and inflammation of the meninges surrounding the brain and spinal cord, and is otherwise known as meningitis. Patients with meningitis can also present with septicaemia, as a result of invading bacteria in the bloodstream. Donovan and Blewitt (2009) identify that meningitis and septicaemia can occur as individual infectious processes or together. This is known as meningococcal disease, and is a life-threatening emergency (Ninis et al. 2010).
During the rapid multiplication of Gram-negative meningococcal bacteria in the blood stream, endotoxin is released from cell membranes, which initiates a number of complex pathophysiological processes, resulting in Gram-negative bacterial sepsis (Ninis et al. 2010). These processes are responsible for the clinical manifestations seen, such as tachycardia, hypotension, tachypnoea and poor oxygen saturations (Table 1). These signs are also attributable to profound septic shock (Bridges and Dukes, 2005).
| Tachycardia |
| Tachypnoea |
| Hypoxia |
| Hypotension |
| Pyrexia and rigors |
| Vomiting |
| Reduced level of consciousness |
| Increased capillary refll time (>2 seconds) |
| Pale, mottled appearance |
| Non-blanching rash |
Endotoxins in the bloodstream activate the immune and inflammatory response, which produces several complex immune processes, resulting in the release of interleukins, inflammatory cytokines, tissue necrosis factor and interferons (Rote and Huether, 2006; Ninis et al. 2010). The release of these substances activates the clotting cascade and coagulation pathways, as a result of damage to the endothelial lining of blood vessels (Ninis et al. 2010). However, Cathie et al (2008) identify that bacteria alter the activity of coagulation pathways, leading to a dysfunction between endothelial cells and blood. This leads to a pro-coagulant state, with platelets and fibrinogen forming clots within blood vessels (Cathie et al. 2008). This is known as disseminated intravascular coaguability (DIC), a complication associated with meningitis (Bone, 1991). The formation of clots, together with increased vascular and capillary permeability, as a result of endotoxins, causes haemorrhage of these clots and leaking of capillaries (Rote and Heuther, 2006; Ninis et al. 2010). Haemorrhage in the capillary beds underneath the skin give rise to the bruised, purpuric rash appearance, classic of meningococcal septicaemia (Figure 1). Ninis et al (2010) recognise that this process can also occur within organ systems, resulting in multiple organ failure and eventual death.
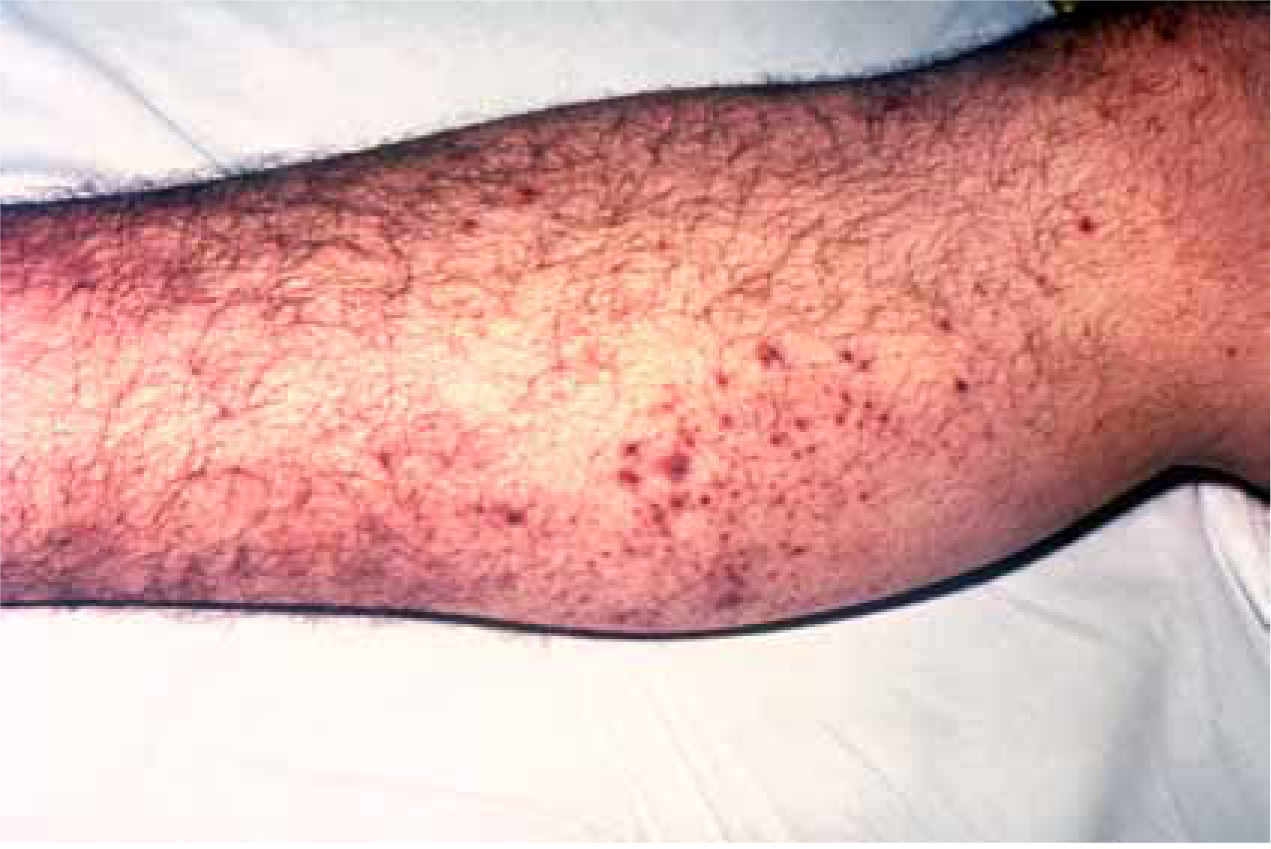
As mentioned, endotoxins increase vascular permeability, which results in a number of complications and cardiopulmonary manifestations. This can lead to the clinical syndrome of septic shock (Ninis et al. 2010). Hypovolaemia occurs due to the normal circulatory fluid leaking through the now permeable vasculature, into interstitial fluid (Cathie et al. 2008). This reduces the venous return to the heart, which leads to a reduction in stroke volume and cardiac output, therefore causing hypovolaemia (Ninis et al. 2010). In response, the heart has to work harder to pump the reduced circulatory volume around the body, thus inducing tachycardia (Cathie et al. 2008). This process continues as more fluid leaks from the intravascular compartments, resulting in worsening hypovolaemia and increased tachycardia. The inflammatory response, induced by endotoxin release, causes myocardial depressant factors to be released, together with vasodilation, therefore adding to the hypovolaemic state and reduction in venous return (Bridges and Duke, 2005). Myocardial depressant factors, in combination with electrolyte imbalance from sepsis, impair myocardial function, resulting in worsening tachycardia and arrhythmia’s (Cathie at al, 2008; Ninis et al. 2010). Tachycardia also increases myocardial oxygen demand, thus increasing respirations to meet demand (Garretson and Malberti, 2007). At cellular level, when supply is insufficient to meet demand, anaerobic metabolism occurs, releasing lactic acid (Marieb and Hoehn, 2007). Lactic acid build-up also leads to increased respirations in order to remove the waste product (Garretson and Malberti, 2007). A raised lactate level may therefore be evident on analysis of blood gases.
Shock, as a clinical syndrome, causes inadequate tissue perfusion, resulting from leakage of intravascular fluid through the capillaries. This causes decreased oxygen delivery in the tissues, therefore inducing hypoxia (Bridges and Dukes, 2005). This manifests clinically as low oxygen saturation levels. Again, this leads to an increase in respiratory rate, in order to compensate for hypoxia (Garretson and Malberti, 2007). Pulmonary oedema is also encountered due to the leakage of fluid through capillary beds within lung tissue, reducing gaseous exchange, adding to hypoxia and tachypnoea (Ninis et al. 2010). Respiratory acidosis as a result of sepsis and impaired gaseous exchange also occurs in worsening septic shock, thus further increasing respiration (Ninis et al. 2010).
It is important to remember there are a number of compensatory mechanisms that attempt to counteract the progression of hypovolaemic shock, including the rennin-angiotensin system to maintain blood pressure, vasoconstriction, and release of vasopressors to increase myocardial contractility and output (Garretson and Malberti, 2007; Ninis et al. 2010). However, in a patient with profound and progressive shock as a result of bacterial sepsis, compensatory mechanisms are unable to cope, and, as a result, the patient rapidly deteriorates.
Boss (2006) identifies fever as a complex response as a result of invading bacterial infection, involving exogenous pyrogens, such as endotoxins. These endotoxins stimulate the pyrogenic activity of endogenous pyrogens such as interleukins, interferons and tissue necrosis factor (Bone, 1991).
These substances increase systhesis of prostaglandin E within the hypothalamus, which initiates and integrates several nervous, endocrine and behavioural pathways. These pathways ultimately cause a rise in core body temperature (Bone, 1991; Boss, 2006). It can also be noted, as Boss (2006) reports, that behavioural activity often results in patients being wrapped up in an attempt to keep warm, thus maintaining a raised temperature.
Ninis et al (2010) highlight that neurological dysfunction occurs due to distribution abnormalities affecting cerebral blood flow and tissue perfusion. Added to this are impaired metabolic function and acidosis from septicaemia, both affecting neurological function. This normally manifests as a reduction in the patient’s Glasgow Coma Scale. Capillary leakage and invasion of the CSF by bacteria also contribute to neurological dysfunction as a result of a rise in intracranial pressure (Boss, 2006).
Long-term effects
Despite being life-threatening, meningococcal septicaemia is survivable through early recognition, aggressive management and appropriate therapeutic interventions. However, literature reports several long-term complications of the disease process, which have devastating impacts on patients (Table 2). Hearing loss is widely acknowledged as the most commonly reported complication (Donovan and Blewitt, 2009; Paul et al. 2011; Peate, 2011). Beckett (2010) reports other complications such as psychiatric, behavioural and learning disabilities, orthopaedic complications, and skin complications. Donovan and Blewitt (2009) identify more serious complications, such as limb loss, as a result of tissue necrosis from coagulation abnormalities and organ failure due to inadequate tissue perfusion from septic shock. Beckett (2010) and the MRF (2003) recognise that in the majority of cases, patients make a full recovery without any residual after effects. However, the references cited above do not go into great detail about the potential complications, with most only listing those that can occur. The National Institute for Health and Care Excellence (NICE) guidelines (2010a) state that all children recovering from bacterial meningitis should undergo a hearing assessment as soon as possible and provide guidance to combat hearing problems.
| Hearing loss |
| Learning disability/long-term mental health problems |
| Physical disability |
| Amputation of one or more limbs |
| Organ failure, e.g. kidney failure |
| Skin conditions |
Pre-hospital management
The pre-hospital management of meningococcal septicaemia focuses on paramedics making an early diagnosis in suspected cases, with rapid transportation to the nearest appropriate facility. This is advocated in NICE guidelines (2010a), the Scottish Intercollegiate Guidelines Network (SIGN) meningitis guideline (2008), and throughout current literature (Cathie et al. 2008; Donovan and Blewitt, 2009; Ninis et al. 2010). Initial management should adopt an airway, breathing and circulation (ABC) approach; maintaining an open airway, applying oxygen and assisting ventilations as required (JRCALC and MRF, 2008), with rapid transport with appropriate pre-alert message passed. JRCALC and MRF (2008) also advise continual reassessment and appropriate management of the patient’s ABCs on route.
Antibiotic therapy
Early antibiotic therapy with benzylpenicillin is recommended for use by paramedic grade clinicians unless this will cause unnecessary delays in transportation, or there is a history of anaphylaxis to penicillin (NICE, 2010b). However, NICE (2010b) stipulate that in order to give antibiotics, a non-blanching rash must be present. It must also be considered that the rash is a relatively late sign of developing sepsis (Ninis et al. 2010).
‘Initial management should adopt an airway, breathing and circulation (ABC) approach; maintaining an open airway, applying oxygen and assisting ventilations as required’
In a study of the symptoms and time of onset in meningitis by Thompson et al (2006), it was found that in 61% of children, the median onset time for the rash was 13 hours. This can have implications for paramedics and patients, as benzylpenicillin may be withheld for a number of hours, by which time prognosis and outcome may be poor.
Cathie et al (2008) present arguments for and against the early use of benzylpenicillin in pre-hospital care. For example, the authors highlight that there is an assumption that in the early stages of the disease process, bacteria are in rapid growth and early antibiotic therapy reduces this bacteria growth (Cathie et al. 2008). However, the JRCALC (2006) benzylpenicillin guideline states that post-administration, the release of toxins may induce hypotension, thus worsening the patient’s condition. JRCALC (2006) provide no evidence to support the statement made. Cathie et al (2008) identify that early antibiotics can cause an increase in endotoxin release, which potentially worsens the disease process. This may manifest in many ways, including hypotension, as stated by JRCALC (2006).
There is a paucity of evidence surrounding the pre-hospital use of benzylpenicillin, and with this in mind current recommendations should be adhered to for the management of these patients, with further research conducted around the use of benzylpenicillin. However, the management guidelines for meningitis produced by SIGN (2008) state that either benzylpenicillin or cefotaxime—an alternative antibiotic therapy—should be commenced, with cefotaxime being the first-line therapy in meningococcal septicaemia. The British National Formulary (Joint Formulary Committee, 2013) supports the use of cephalosporins such as cefotaxime where there is known penicillin allergy, or where benzylpenicillin is not readily available. This raises the question of whether or not paramedics should be able to administer cefotaxime for suspected meningococcal septicaemia, as an alternative to benzylpenicillin. There is no pre-hospital evidence to suggest this would be beneficial and the cost of a single dose of cefotaxime is more than double that of a single dose of benzylpenicillin (Joint Formulaty Committee, 2013). Before any changes in therapeutic interventions, the number of cases of meningococcal septicaemia encountered by paramedics must be considered, together with the advantages, disadvantages and cost implications of drugs.
Fluid resuscitation
In the shocked patient, fluid resuscitation should be commenced, with bolus of 20 ml/kg in children and 250 ml in adults, given en route to hospital (JRCALC and MRF, 2008). At the time of publication, Cathie et al (2008) and Lighthall and Pearl (2003) identified little documented evidence to suggest that a crystalloid fluid is preferential over a colloid fluid in the pre-hospital management phase, nor any evidence to suggest one crystalloid is better than the other. However, this has now been contradicted by Lodha et al (2011), who suggest crystalloids as the preferred choice of fluids for the management of septic shock. The use of crystalloids in fluid resuscitation is advocated in the management guidelines produced by JRCALC and MRF (2008), and current paramedic practice is to administer normal saline for fluid replacement (JRCALC, 2006). Continual reassessment of the patient’s circulatory status should be undertaken during transport to hospital, with further fluid boluses as required to correct hypotension and maintain perfusion (NICE, 2010a). Blood glucose measurements should also be monitored and corrected as required, following JRCALC (2006) guidelines.
In-hospital management
The initial stages of in-hospital management for suspected cases of meningococcal septicaemia are similar to that carried out in the pre-hospital phase (NICE, 2010a), with emphasis given to early assessment of ABCs, signs of shock, signs of raised intracranial pressure, and diagnostic testing to identify causative organisms, with early administration of antibiotics and intravenous fluid replacement.
Antibiotic therapy
The antibiotics that are recommended for use within hospital are ceftriaxone or cefotaxime (Ninis et al. 2010). The author has found little evidence to suggest that these antibiotics are preferential over benzylpenicillin for the initial management of meningococcal septicaemia. However, Cathie et al (2008) suggest that while penicillins are effective against bacteria, a broader spectrum antibiotic should be used until bacterial cultures can confirm the causative organism. Therefore, it can be considered that ceftriaxone and cefotaxime have a broader spectrum of action than that of benzylpenicillin. Care must be taken when using ceftriaxone, as the drug can have adverse effects when given alongside calcium-containing infusions (Cathie et al. 2008). The advocated drug of choice is cefotaxime, which as previously discussed could potentially have a role in pre-hospital care.
Fluid resuscitation
Fluid resuscitation remains a key priority, as outlined within NICE (2010a) guidelines. As within pre-hospital care, the fluid of choice is a crystalloid. Consideration should also be given to the use of albumin solution for persistent, decompensated shock (NICE, 2010a). Further management of persistently shocked patients includes the use of anaesthesia and mechanical ventilation with intensive care admission (Cathie et al. 2008; Ninis et al. 2010). The main aim of mechanical ventilation is to reduce tachypnoea and therefore improve tissue oxygenation (Ninis et al. 2010). Anaesthesia of such patients requires careful consideration, and should only be carried out by appropriately trained and competent staff. This is advocated within NICE (2010a) and JRCALC and MRF (2008) guidelines, which state discussion with intensivists and other specialists, e.g. anaesthetists and paediatricians, is essential.
Inotropic support and other interventions
There are a number of other therapeutic interventions that can be used in patients with meningococcal septicaemia, including the use of inotropic support, steroid replacement, and other experimental therapies such as activated protein C (Cathie et al. 2008). However, experimental therapies are not yet supported with clinical trials and lack evidence to support their use, and as such are not supported in the NICE (2010a) guidelines. In light of this, experimental therapies have not been discussed in great detail in this article.
‘Experimental therapies are not yet supported with clinical trials and lack evidence to support their use’
The aim of inotropic support in persistently shocked patients is to improve and maintain tissue perfusion and oxygenation by improving myocardial contractility and function. This increases cardiac output, which in turn raises blood pressure (Cathie et al. 2008). However, patients presenting with DIC have a reduced circulatory volume, which is the cause of reduced cardiac output. Therefore, inotropes must be given in conjunction with fluid boluses in an attempt to restore the patient’s circulatory volume. The NICE (2010a) guidance has a poor approach in recommending which inotrope to use, and states that clinicians should follow local or national protocols. Cathie et al (2008) identify and discuss the benefits and limitations of a number of inotropes for use in meningococcal septicaemia, such as dopamine and adrenaline. No one particular drug is advocated and local protocols should be adhered too for the use of inotropic support.
Steroid replacement therapy
Steroid replacement in paediatrics appears controversial in the management of meningococcal septicaemia. In the NICE (2010a) recommendations, low-dose corticosteroid replacement is only advised where the patient remains unresponsive to inotropes and under the direction of a paediatric intensivist. However, a Drug and Therapeutics Bulletin (DTB) (2007) highlights that low-dose steroids reduce inflammation caused by the immune response to infection, and can reduce long-term neurological complications (DTB, 2007). This is supported by Cathie et al (2008), who also counter argue that steroids are being used in increasing frequency without any evidential support. It therefore must be considered that the use of steroid replacement therapy in meningitis is emerging and requires further research before advocating its use as a first-line management option.
Conclusions
This article has considered the relevant pathophysiology, aetiology and disease processes relating to meningitis and meningococcal septicaemia. Current UK pre-hospital management options have been discussed, with potential changes in antibiotic therapy identified. In addition, it has highlighted that paramedics are only able to administer benzylpenicillin in the presence of a non-blanching rash, which is a late sign of the disease. With in-depth research, it may be justifiable to use benzylpenicillin in clinically suspected cases of meningococcal septicaemia without the presence of a rash, to initiate early therapy. The hospital management of this patient group is the same as that in the pre-hospital phase, with the addition of adjunctive therapies, such as anaesthesia and mechanical ventilation. Other potential adjunctive therapies and treatment options require further research before recommendations can be made. It is essential for all clinicians, whether pre-hospital or hospital based, to adopt a high index of suspicion of meningitis, to recognise cases and commence treatment at the earliest opportunity.
