Incidents of beta blocker overdose and beta blocker toxicity are associated with significant morbidity and mortality (Sheppard, 2006). Prehospital care providers are likely to have experience and knowledge of managing patients who present following accidental and intentional drug overdose.
However, the incidence of beta blocker overdose is less frequent than other commonly prescribed medications in the UK (Health Protection Agency, 2010); and due to a broad range of presenting symptoms from asymptomatic through to life-threatening cardiogenic shock, managing patients following beta blocker overdose is likely to be both clinically challenging and complex (Page et al, 2009).
This case report examines an intentional overdose of propranolol, and its subsequent treatment with intravenous glucagon. The pharmacology of beta blockers and toxicity in overdose will be examined, as will management of this condition, including glucagon treatment.
Finally, as UK paramedics are familiar with administering intramuscular glucagon for the treatment of hypoglycaemia have this drug available to them, and possess the necessary skills for obtaining intravenous access (Joint Royal Colleges Liaison Committee (JRCALC), 2006); could intravenous glucagon be considered appropriate for administration by UK paramedics in cases of symptomatic beta blocker overdose?
Case presentation
A 32-year-old male presented to paramedics approximately one hour following an intentional overdose of propranolol. Patient history and evidence on scene indicated ingestion of 2.4 g (sixty 40 mg tablets), with an unknown quantity of alcohol. On arrival, the patient was alert and orientated, with a clear and self-maintained airway, respiratory rate of 14 breaths per minute, heart rate of 64 beats per minute, and a Glasgow coma scale (GCS) of 15 (eyes 4; verbal 5; motor 6).
Initial examination indicated blood pressure of 115/90 mmHg, temperature of 36.9 °C, blood glucose level of 5.6 mmol/L, and a peripheral capillary refill time of approximately 2 seconds. SpO2 recording indicated an oxygen saturation of 96% on air, and 12-lead ECG revealed sinus rhythm with normal PR, QRS, and QT intervals. The rest of the physical examination was normal.
The patient's previous medical history included two intentional drug overdoses, depression and anxiety. Current medication included propranolol 40 mg once daily and citalopram 20 mg once daily.
The patient was in paramedic care for approximately one hour. During this period, the patient's heart rate decreased to 60 beats per minute, with a decline in blood pressure to 74/52 mmHg, and a decline in GCS to 13 (eyes 3; verbal 4; motor 6).
Prehospital management included supportive measures, intravenous access, an intravenous fluid bolus of 250 ml sodium chloride 0.9% solution, and a hospital pre-alert message. At hospital handover, despite some clinical improvement, the patient remained hypotensive at 88/58 mmHg. Emergency department treatment included a large bolus dose of intravenous glucagon, followed by glucagon infusion, which produced a dramatic improvement in the patient's condition.
Discussion with the department's staff indicated that intravenous glucagon is used as first-line treatment and antidote in symptomatic beta blocker overdose (Joint Formulary Committee, 2011; National Poisons Information Service, 2011a; 2011b). On reflection, a question became apparent; as glucagon was available to the paramedics on scene, would it have been beneficial to have administered this drug intravenously as part of the treatment for symptomatic beta blocker overdose prior to hospital arrival?
Beta blockers: background and pharmacology
Beta-adrenoceptor blocking drugs, more commonly known as beta blockers, are a group of drugs used in the treatment and management of angina; hypertension; congestive heart failure; arrhythmias; reduction of post-myocardial infarction mortality; thyrotoxicosis; migraine prophylaxis; glaucoma; essential tremor, and anxiety (Joint Formulary Committee, 2011). Various types of beta blocker are currently used in UK practice, each with some variation in function (Table 1).
At therapeutic doses, beta blockers work by selectively antagonizing, or blocking, the body's beta-adrenergic receptors, of which there are three known types: beta-1, beta-2, and beta-3 (DeGeorge and Koch, 2007).
Beta-1 receptors primarily reside in myocardial tissue, and stimulation of these receptors leads to an increase in intracellular cyclic adenosine monophosphate (cAMP), creating an increase in heart rate, myocardial contractility, and atrioventricular node conduction (Lohse et al, 2003). Beta-2 receptors are found primarily in bronchial and peripheral smooth muscle, and stimulation results in smooth muscle dilatation (Wax et al, 2005). Beta-3 receptors are less well defined, but are thought to reside in adipose and myocardial tissue and exert influence on lipolysis and cardiac contractility (Sheppard, 2006).
Thus, beta blockers are therapeutically used to antagonize beta-adrenergic receptors, thereby blocking the effects of catecholamines on these receptors and reducing intracellular cAMP, resulting in a desired decrease in cardiac contractility, heart rate, and blood pressure (Wax et al, 2005).
Beta blockers: toxicity, toxic doses and clinical features
Toxic doses and individual responses to overdose vary greatly, and clinical features will depend on the specific drug ingested; whether the drug is immediate or sustained release; the dose and timing of overdose; co-ingestions; patient co-morbidities; and patient intent (Mokhlesi et al, 2003; Wax et al, 2005). In addition, patients with underlying cardiovascular disease, such as cardiac conduction disturbances or patients taking other cardiac medications such as calcium channel blockers, are likely to be particularly vulnerable to beta blocker toxicity (Bledsoe and Clayden, 2005; Wax et al, 2005).
Table 1 lists the lowest reported oral toxic doses of beta blocker overdose (where beta blockers were the sole pharmacological agent) presented in medical literature, and also current UK suggested toxic doses for adults and children.
Clinical features of beta blocker toxicity are effectively an extension of their pharmacology, with the most significant effects influencing the cardiovascular system (DeWitt and Waksman, 2004). Although specific symptoms vary, common features include hypotension and bradycardia, in addition to cardiac conduction abnormalities, including first or second degree atrioventricular block, congestive heart failure with or without pulmonary oedema, syncope and cardiogenic shock (Mokhlesi et al, 2003; DeWitt et al, 2004).
Beta-adrenergic selectivity is less apparent in overdose, therefore beta-1 selective drugs may block beta-2 and beta-3 receptors, causing systemic effects of beta-blockade such as bronchospasm and reduced cardiac contractility (Sheppard, 2006; National Poisons Information Service, 2011b).
Lipid solubility (lipophilicity) of the drug is also an important factor in overdose. Highly lipid-soluble beta blockers, such as propranolol, are able to cross the blood-brain barrier resulting in an increased likelihood of neurological symptoms in toxic doses, such as drowsiness, confusion, hallucinations, seizures, and coma (DeWitt et al, 2004; Wax et al, 2005). However, if the brain is poorly perfused due to hypotension, drowsiness and confusion may also present (Sheppard, 2006).
Certain beta blockers also antagonize cardiac sodium channels, prolonging the QRS interval and increasing the risk of ventricular arrhythmia, which may increase toxicity in overdose (Sheppard, 2006). Other manifestations include hypoglycaemia and hypocalcaemia, although these are less common (Mokhlesi et al, 2003; National Poisons Information Service, 2011b).
Prehospital management of beta blocker overdose
Prehospital care providers are likely to be presented with patients with various manifestations of beta blocker overdose (Bledsoe and Clayden, 2005); therefore management will depend upon the severity and extent of symptoms displayed, with an awareness and preparation for potential patient deterioration.
Prehospital care is likely to involve supportive measures of the patients airway, breathing and circulation, plus monitoring of respiratory rate, heart rate, blood pressure, cardiac rhythm, blood glucose, and temperature. Intravenous access is likely to be required at an early stage.
In addition, therapies to support perfusion are often needed (Sheppard, 2006). Hypotension may respond to intravenous fluid therapy, and prehospital intravenous fluid management should be administered as per normal guidelines (JRCALC, 2006). However, it should be noted that hypotension in beta blocker overdose is mainly due to decreased myocardial contractility, and not fluid depletion or misplacement. As a result, large quantities of intravenous fluids should be used with extreme caution (Mokhlesi et al, 2003).
Symptomatic bradycardia should be managed with intravenous atropine, as per normal guidelines (JRCALC, 2006). However, the effectiveness of atropine against symptomatic bradycardia in beta blocker overdose has been questioned, and often transient clinical improvement is seen, followed by return of bradycardia despite maximum dose (Sheppard, 2006; Wyatt et al, 2006). Nebulized salbutamol, as per normal guidelines, may be of benefit in bronchospasm (JRCALC, 2006; National Poisons Information Service, 2011b) (Table 2).
| Adult | Paediatric | |
|---|---|---|
| Fluid management Sodium chloride 0.9% (IV) | 250 ml boluses Maximum 2 litres | 20 ml/Kg (Initial bolus) |
| Atropine (IV) | 500 mcg (Initial dose) Maximum 3 mg | 40 mcg/kg* Maximum 3 mg |
| Salbutamol (nebulized) | 5 mg | > 6 years - 5 mg |
*Paediatric atropine doses as per Joint Formulary Committee (2011)
Glucagon treatment for beta blocker overdose
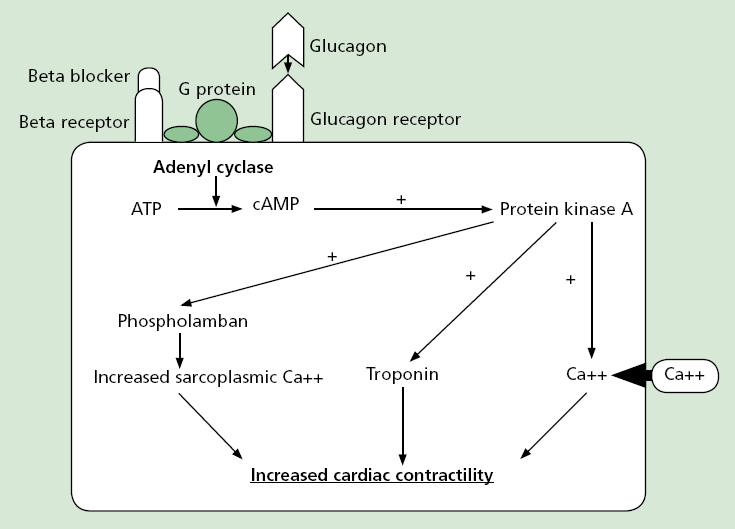
Glucagon is a regulatory hormone produced and secreted by the alpha cells of the pancreatic islets of Langerhans (Kerns, 2007). In beta blocker overdose, glucagon is thought to bypass blockaded beta adrenergic receptors by directly stimulating separate receptor sites; a G-protein on the beta receptor complex, which activates adenyl cyclase in cardiac tissue in a similar way to beta adrenergic receptor stimulation, generating the conversion of adenosine triphospate (ATP) to cyclic adenosine monophosphate (cAMP (Sheppard, 2006; Kerns, 2007).
The resulting activation of cAMP mediated activation of protein kinase A, which leads to phosphorylation of three intracellular protein systems, results in an increase in sarcoplasmic calcium, troponin, and increased intracellular calcium flow (Erdman, 2004) (Figure 1). As a result, glucagon demonstrates positive inotropic and chronotropic effects, causing an increase in strength and rate of myocardial contraction despite beta adrenergic receptor blockade (Mokhelsi et al, 2003; Kerns, 2007).
Intravenous glucagon is considered both an antidote and first-line treatment in symptomatic beta blocker overdose (Joint Formulary Committee, 2011; National Poisons Information Service, 2011a; 2011b). The recommended adult dose is an initial intravenous bolus of 2–10 mg (paediatric: 50–150 mcg/kg, to a maximum of 10 mg), administered slowly over 10 minutes, followed, if required, by an infusion of 50 mcg/ kg/hour (Joint Formulary Committee, 2011; National Poisons Information Service, 2011a).
Glucagon works rapidly, with improvements in heart rate, blood pressure, cardiac contractility and output within minutes of an initial dose (Mokhlesi et al, 2003; Sheppard, 2006; Kerns, 2007). However, in severe toxicity, glucagon may have a transient effect requiring large quantities to be infused, with the possibility of a patient requiring approximately 50 mg over a 24-hour period (Wyatt et al, 2006). Case reports have even stated dosages administered of over 440 mg in one patient (Kerns, 2007).
Glucagon is associated with certain side-effects, most commonly nausea and vomiting, becoming increasingly apparent with larger doses (Kerns, 2007). This can be problematic in patients with reduced consciousness, and needs to be anticipated with appropriate patient positioning, airway protection and management (Wyatt et al, 2006). Anti-emetics may also be of benefit in this instance (Erdman, 2004).
In addition, due to glucagon's stimulation of glycogenolysis, transient hyperglycaemia is a likely adverse effect of glucagon use in beta blocker overdose; however, it typically does not require intervention (Kerns, 2007).
Is glucagon evidence-based?
The cardiovascular effects of glucagon in the presence of beta adrenergic blockade was first reported during the 1960s (Parmley et al, 1968), and the first human case of beta blocker overdose treated with glucagon was reported in 1971 (Kosinski et al, 1971).
Numerous case studies have reported clinical improvement after glucagon treatment for symptomatic beta blocker overdose (Peterson et al, 1984; Khan and Miller, 1985; Smith et al, 1985; O'Mahoney et al, 1990; McVey and Corke, 1991; Ehgartner and Zelinka, 1998; Love et al, 1998; Sheppard, 2006; Fung et al, 2007; Kerns, 2007).
However, despite the large number of published case reports supporting the effectiveness of glucagon in beta blocker overdose, fewer reports have been published where glucagon was the sole pharmacological agent used (Smith et al, 1985; Love et al, 1998; Kerns, 2007).
In addition, several published reports also detail glucagon's failure to produce clinical improvement (Bailey, 2003; Sheppard, 2006; Kerns, 2007). However, variability in response to glucagon is likely, due to the wide ranging factors involved in beta blocker overdose, such as type of drug ingested, amount, patient specific factors, and timing of ingestion and therapy (Bailey, 2003; Sheppard, 2006). In addition, glucagon forms just one part of a broad and complex treatment regime (National Poisons Information Service, 2011b).
No human randomized controlled trials of glucagon treatment following beta blocker overdose have been published (Boyd and Gosh, 2003; Kerns, 2007). As a result, the evidence in support of glucagon comes from animal studies and case reports (Bailey, 2003), which could be argued to be insufficient evidence to justify its use (Boyd and Gosh, 2003).
However, it could equally be argued that since glucagon has been used successfully in the treatment of symptomatic beta blocker overdose since the 1960s, and is regarded as both antidote and first-line treatment in beta blocker toxicity, to investigate its effectiveness against any other treatment regime and potentially deprive a patient of a life-saving intervention would be unethical (Lee, 2004; O'Connor et al, 2005).
Finally, although in-hospital use of glucagon in beta blocker overdose is well documented, no studies exist addressing the effectiveness or safety of glucagon administered by paramedics in prehospital treatment and management of symptomatic beta blocker overdose (Wax et al, 2005). In UK prehospital paramedic literature, only one reflective case report exists regarding glucagon used in the management of symptomatic beta blocker overdose, and in this instance, glucagon was not administered by paramedics prior to hospital arrival (Durham, 2008).
Glucagon treatment for beta blocker overdose: a prehospital intervention?
Glucagon is currently available to UK paramedics as a 1 mg intramuscular injection for treatment of hypoglycaemia (JRCALC, 2006). The current presentation, also suitable for intravenous use, is prepared as a sterile white powder in a 2 ml vial, accompanied with a disposable prefilled syringe containing 1 ml sterile water for reconstitution (Novo Nordisk, 2011).
Typically, a single paramedic may only have access to 2–4 mg of glucagon. However, if a rapid response vehicle and double crewed ambulance are responded to the same incident, which would be more likely in the case of symptomatic beta blocker overdose, a total of 4–8 mg of glucagon would be available on scene. This quantity could be sufficient for an initial intravenous bolus dose, or with a small increase in the quantity carried, the maximum dose of 10 mg could be achieved. In addition, paramedics already possess experience of administering glucagon, and are capable of managing the associated side-effects of its use. Thus, it would seem plausible that early paramedic administered intravenous glucagon in cases of symptomatic beta blocker overdose could be a possibility.
However, issues with increased cost of drug stocking and wastage, additional education and training requirements for paramedics, and lack of robust evidence of beneficial response to glucagon use in beta blocker overdose are some of the initial drawbacks to possible changes in practice. Future research would need to focus on the safety and efficacy of paramedic administered intravenous glucagon for symptomatic beta blocker overdose if evidence-based practice is to be adhered to.
Perhaps current best practice for UK paramedics would be to have an awareness of the indications and implications of glucagon use in beta blocker toxicity, and should a patient present in extremis following beta blocker overdose, early discussion with the receiving emergency department's staff should take place with the view to administering an initial bolus dose of intravenous glucagon, should paramedic knowledge, experience, and drug supply allow.
Conclusion
Incidents of symptomatic beta blocker overdose are rare, but life-threatening. Despite a lack of robust evidence in support of its use, glucagon remains an important and established part of the management and treatment of these patients. The option of early paramedic administered intravenous glucagon, if knowledge, experience, and drug supply allow, could be a useful adjunct in the management and treatment of symptomatic beta blocker overdose. However, changes to UK paramedic practice will ultimately require further, more specific research.
