This narrative review aims to add evidential clarity to the ongoing debate surrounding the NHS-mandated bare-below-the-elbows (BBE) policy. Specifically, the present review focuses on the context of ambulance clinicians working in the out-of-hospital environment and their wristwatch use during clinical activity.
Fundamentally, the BBE policy aims to increase the efficacy of handwashing practices by reducing the potential for pathogens to remain on the hands on jewellery, false nails, watches and long sleeves.
In relation to watches, this policy is practicable when working in conventional clinical environments such as hospitals. Here, clocks are numerously available for the purposes of recording temporal data and maintaining situational awareness; clinical devices are almost universally used for recording observations. This is not the case in the out-of-hospital environment. The policy therefore poses additional challenges for clinicians working in unplanned and emergency care settings.
While alternative time devices are available, such as fob watches, tablet computers and mobile phones, these have been demonstrated to pose an equal, if not greater, microbial risk to patients than any wristwatch due to the need to touch such devices to operate them (Bhusal et al, 2009; Jeans et al, 2010; Mark et al, 2014; Raza et al, 2017). This need to touch such items to use them as part of temporal record-keeping may result in a heightened cross-contamination risk of healthcare-acquired infections (HCAIs).
While numerous studies have examined BBE in the context of white coats and shirtsleeves, comparably little evidence surrounds the risks posed by the wearing of wristwatches and using an alternate time device. The studies that do address this matter report varying conclusions of differing quality, and study methodologies often use inconsistent definitions of BBE.
Literature search
Two searches by the Library and Knowledge Service for Ambulance Services in England were commissioned by the author. This service undertakes searches of PubMed, MEDLINE, CINAHL, EMBASE and EMCARE databases.
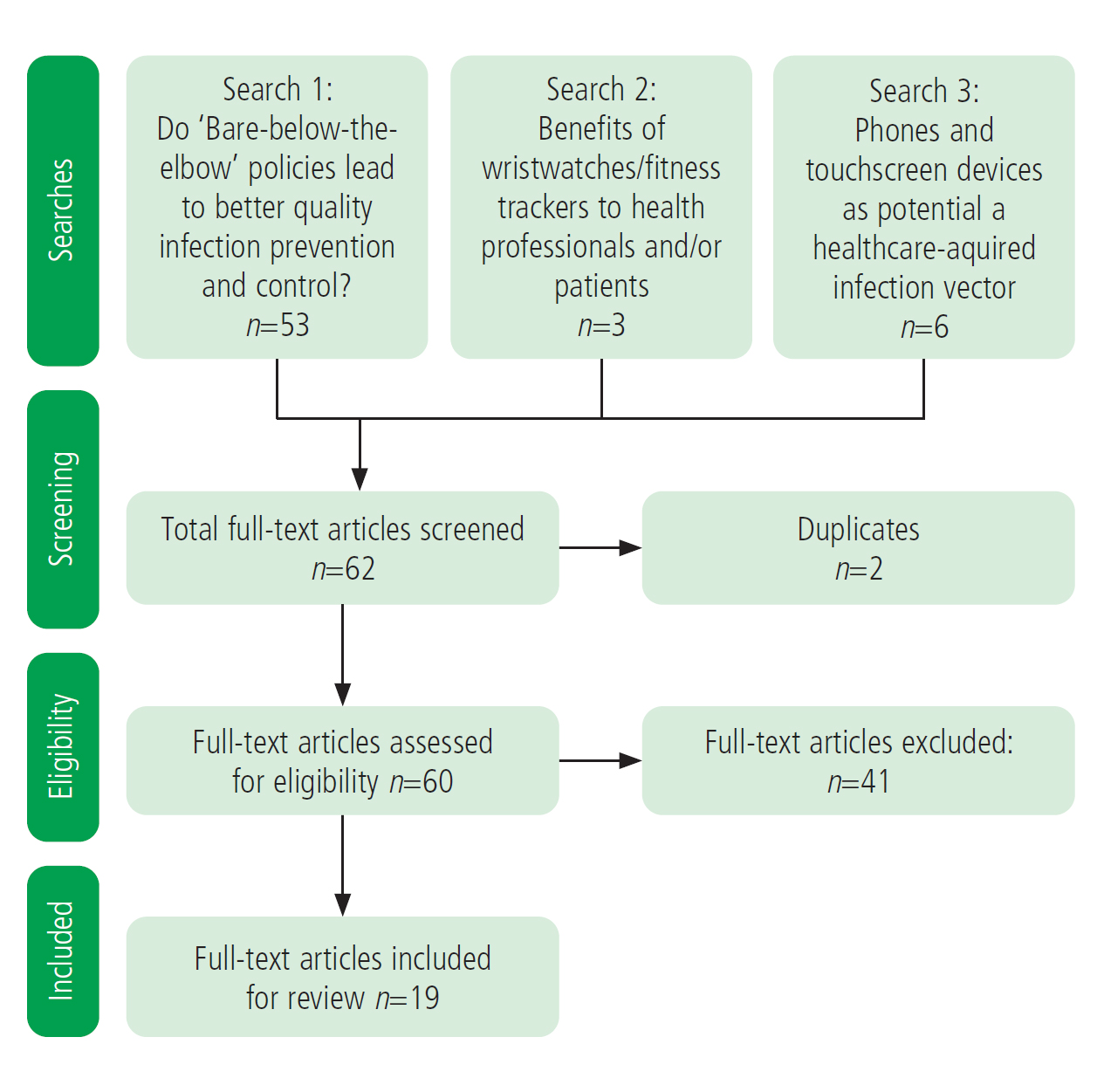
The first search aimed to find literature around whether BBE policies and the absence of wristwatches led to better-quality infection prevention and control. This focused on keywords which included: bare below the elbow; wristwatch wearing in healthcare; wrist cleaning; wrist contamination; quality of hand washing; hand contamination; and wrist contamination. This search returned 53 papers.
The second search focused on whether wearing a wristwatch enabled better healthcare provision and whether there were any other benefits to health professionals or their patients from wearing watches/fitness trackers. This search returned three studies.
A further search was then conducted to examine the HCAI vector risk posed by phones and touchscreen devices. This search returned six papers.
These papers were then manually screened against an inclusion criterion that studies had to relate to the wearing of a wristwatch in the context of an HCAI outcome measure. Studies solely focused on long sleeves were excluded.
Figure 1 shows the systematic review flowchart; a stratified table of studies is available in Appendix 1 (online).
Discussion
Impact on handwashing
As part of their guidance surrounding HCAIs, the National Institute for Health and Care Excellence (NICE) (2017) advocates a BBE policy during engagement in clinical practice to ensure that hands can be properly decontaminated. In a rare occurrence of basing recommendations on minimal/inadequate evidence, on the part of NICE, the work of Farrington et al (2010) is the only evidence cited in support of this guidance.
Farrington et al (2010) examined 159 hospital doctors and medical students. They found no difference between BBE participants and those who were not bare (NB) when area of hands missed in hand washing were examined (P=0.37; 95% CI [−3.17, 1.17]), but did identify that NB participants were more likely to miss areas of wrist (P<0.01; 95% CI [−24.77, −3.03]). Farrington et al (2010) conclude from this finding that being bare below the elbow makes no difference to the quality of handwashing, only to the quality of wrist washing, and that the clinical significance of the quality of wrist washing in relation to the transmission of HCAIs is unknown. This remains so according to the present literature search as there is still no study that has directly looked at the quality of wrist washing on HCAI transmission.
Farrington et al's (2010) study is limited by its small sample size, as well as its inclusion of only doctors and medical students. A plain wedding band was also allowed to be worn within the BBE cohort in this study, but this does not seem to have influenced the study results or outcome. The potential for a change of behaviour during examination could also have influenced participant performance (the Hawthorne effect). Expected detail regarding participant recruitment, randomisation and cohort size was also not reported. As with all other studies that will be examined, this study is also limited in relation to its prehospital context applications as it was conducted in a hospital.
Similar results were identified in an observational study of 92 hospital-based doctors by Willis-Owen et al (2010). This study identified no statistically significant differences in the presence of clinically significant pathogens or multidrug-resistant organisms between the 49 BBE and the 43 NB participants. Analysis regarding sex, grade and specialty were also performed with no significant findings.
While this study had a small sample size, the authors mitigated against a type 2 error by using a power calculation to obtain 0.8 power with an alpha of 0.05 to find a 10% difference in level of hand hygiene.
In this context, a type 2 error refers to accepting the null hypothesis (as the authors have done here) when the experimental hypothesis—increased presence of clinically significant pathogens in one of the two conditions—is correct (Serdar et al, 2021). This is described as a false negative. The risk of this is higher in a study with a smaller sample size as you may not gather enough samples to gain a representative sample of the target population (which, in this case, is hospital doctors), and a resultant noticeable difference between the two conditions. Power is the probability of incurring a type 2 error. In this study, which used a power of 0.8, there is a 20% chance of a type 2 error and accepting a false-negative result.
Regarding the alpha value, this is an indication of the risk taken by the researcher(s) that their finding is not applicable to the full population sampled. In other words, the researchers have accepted their experimental hypothesis when the null hypothesis is true (Serdar et al, 2021). This is described as a false positive. The most commonly used alpha value is 0.05, which is a 5% chance of incurring a false-positive result. Studies can often report ‘higher’ alpha values, such as 0.01—a 1% chance—and even 0.001—a 0.1% chance of a false positive. The smaller the alpha value achieved and reported (normally indicated by a ‘P value’), the more confident you can be in a finding applying to the full population of a sample. The observational format and opportunity sample method of doctors only, along with opportunity for the Hawthorne effect, are also limitations.
Burger et al (2011) identified significant colony reduction between 66 hospital-based BBE (n=38) and NB (n=28) doctors after washing all areas of the hand and wrist except for the NB nondominant (left) palms and wrists. The left and right forearms of both BBE and NB groups failed to reach significant colony number reduction following handwashing. However, as highlighted by Griffin et al (2011), it is unclear what was passable as a BBE dress state in this study as rings and watches may have been allowed in both participant groups. This is a deep confounding factor of this study and its findings.
Fagernes and Lingaas (2011) undertook detailed multivariate analysis of the factors affecting the hand microbiology of 465 Norwegian hospital-based health workers across two study periods in 2004 and 2007. This study identified that participants wearing wristwatches had three times as many bacteria on their hands as non-wristwatch wearers (unadjusted effect estimate: 5.70; 95% CI [3.04–10.68]; P<0.001; adjusted effect estimate: 3.25; 95% CI [1.73–6.07]; P<0.001]). However, while this paper is data-rich, the analysis relating to wristwatches is weakened by the second study period in 2007 tracking the presence of wristwatches (n=200), during which time a more sensitive detection method was used. These data cover fewer than half of the study participants, with vastly unequal cohort sizes, where the number of participants not wearing a wristwatch (n=121) was nearly double the number of those who were (n=79), with further opportunity for skewed results resulting from insufficient statistical power. The authors demonstrated awareness of this possibility, having referred to this in relation to decorative (n=10) and multiple (n=50) rings, but did not consider it relevant for the analysis relating to wearing wristwatches (n=79). While the authors performed separate analyses to try to mitigate for these factors, it is unclear whether this is sufficient to provide meaningful analysis.
In addition, Fagernes and Lingaas (2011) explored the possibility that the uncontrolled wristwatch variable in the initial 2004 study period may account for the raised bacteria count on hands where finger rings were present (P=0.002). This would seem inconsistent with the 22 studies they cite in their own discussion on this topic, which highlight an association between a higher bacterial load and wearing rings. This consideration is further strengthened by their own analysis into the carriage of non-fermentative Gram-negative rod species. Here, the authors identified a significant unadjusted effect estimate for hands with wristwatches (OR 2.21; 95% CI [1.21–4.03; P=0.01), which then disappears when finger rings and nail polish are adjusted for in the final model (OR 1.34; 95% CI [0.64–2.81]; P=0.442). This illustrates that rings and nail polish pose an equally significant, if not greater, risk as wristwatches to the carriage of harmful bacteria. While this paper no doubt adds to the total evidence on this topic, its impact is questionable once again because of a small sample size and weaknesses within the study design and results analysis.
Szumska et al (2022) had presented the most contemporary evidence on this matter at the time of writing. This study is vast, undertaken across 123 Polish healthcare facilities over two separate study periods in 2017 and 2018, collating data from 7544 participants who were directly involved in patient care. It was undertaken alongside an educational campaign entitled ‘Close the door to hospital infections’ sponsored by Medilab, the Polish Scientific Associations and the scientists of Medical University of Bialystok.
Szumska et al (2022) used an ultraviolet camera to compare hand disinfection between an initial enrolment and a subsequent enrolment, which followed an informal education campaign around hygiene best practices. Correct hygiene as assessed as having >94% of the hand covered by the fluorescent solution following decontamination. Correct hand hygiene was demonstrated in 4879 participants (64.7%) but not in 2665 (35.3%). This study is, once again, data-rich with numerous descriptive findings drawn from the data, but minimal inferential analysis was performed that is relevant to the theme of the present review. Regarding participants who incorrectly decontaminated, 1103 (41.4%) wore wristwatches and 1243 (46.6%) wore rings. Although the authors note within their conclusion that their study did not examine removal of rings and watches to improve hand hygiene, the comparative data between correct and incorrect BBE preparation has not been published, nor explored meaningfully, in this paper.
Notably, when the ‘time of participation’ data is scrutinised, it shows that the percentage of participants correctly disinfecting their hands drops from 65.4% (n=4431) to 58.0% (n=448) between the two enrolment points. This is an interesting finding given the context of the study, which ran alongside an infection prevention and control education campaign to improve hand hygiene. These data would suggest that this campaign has not been successful.
While the sample size was large and an established and robust study protocol was implemented, the results have not been explored beyond the role and clinical seniority of those who did not correctly cleanse their hands, along with the associated risk factors present. This is a huge missed opportunity to better understand the sources of contamination for these cohorts.
There are also major differences in size between the first (n=6772) and second (n=772) cohort. However, Szumska et al's (2022) analysis seems congruent with the intended aim, which focused on the role and effectiveness of education in relation to improving hand hygiene in hospitals. This has implications for NHS BBE policy as education on improvement of hand hygiene did not have the desired effect in this case.
Bacterial contamination of watches
The next theme to be considered as part of this review relates to bacterial contamination of items such as watches and other time devices. Field et al (1996) aimed to identify the bacteria, which were sampled from under the watches and rings of 20 dental surgeons and 20 non-clinical participants. This study revealed that clinical staff harboured greater numbers of bacteria in these areas than the control group, but these bacteria were unlikely to cause oral infections, although they could pose threats to immunocompromised patients. This study had a small sample size and is now quite dated. It also provides limited information relating to the type of bacteria that were recovered, despite the interpretation from the authors. It was also carried out within a dental context.
Jeans et al (2010) provided a more contemporary example of the previous study by comparing skin swabs of the wrists of 100 hospital-based watch-wearing (n=52) and non-watch-wearing (n=48) health professionals. In the first of two cross-sectional cohort studies, they identified Staphylococcus aureus on the wrist on 25% of participants wearing a wristwatch and on 22.9% of those who did not and concluded that watch wearers had more bacteria on their wrists but not on their hands (P<0.001). This finding is concordant with that of Farrington et al (2010) and adds further depth to this topic. The second study builds on this finding by demonstrating that removing the watch before sampling led to greater contamination of the hands compared to non-watch wearers (P<0.001) (Jeans et al, 2010). This is a relevant consideration in relation to the use of alternative time sources such as mobile phones and fob watches, which are utilised in the absence of a watch. This is explored further on.
This was investigated by Bhusal et al (2009), who examined the bacterial colonisation of the wristwatches of 100 hospital-based multidisciplinary health personnel. This robust methodology, which examined the pattern of wear and longevity (total length of time the watch has been worn for) of the swabbed watch revealed that 78% of watches swabbed were colonised by typical skin flora considered to be of minimal risk to patients. Only one watch grew a potential HCAI pathogen. This study therefore highlights that watches may be less of a risk to patient health than policy makers suggest.
This study would have benefited from a larger sample but used an innovative approach to the topic by including the descriptive statistics of the watch-wearing behaviour of the participants.
Bacterial contamination of phones and touch screens
Jeske et al (2007) used a repeated-measures design to explore the contamination of the hands of 40 anaesthetists by both fixed and personal mobile phones within an operating theatre following a short phone call. They identified that personal mobile phones (n=38) carried a much greater risk of contamination than fixed phones (n=33), with both showing an equal chance of contamination with human pathogenic bacteria (n=4 in each group for a total of eight cases). This innovatively applied study design reduces the impact of participant variables; however, order effects were not controlled, with the mobile phone always used first. Despite the in-hospital setting, this nonetheless demonstrates that mobile phones are capable of being a contamination vector for HCAIs, which is a valuable conclusion in the context of this review.
This issue was taken one step further by Mark et al (2014), who investigated the level of contamination on 50 smartphones used by doctors and allied health professionals within a hospital. This was supplemented by a questionnaire, handed out to 150 health professionals, which aimed to investigate the level of smartphone use at work. This study found that 62% of phones (n=31) had three bacterial colonies or fewer, and no pathogenic or antibiotic-resistant strains were cultured. The questionnaire found that 88% (n=132) of participants used their phone within the workplace, 55% (n=48) of whom used their phone for clinical reasons. Only 37% (n=49) said they regularly cleaned their phone. This well-designed study adds further evidence that smartphones can be a vector for bacteria but that such bacteria is minimally harmful to patients in most cases. However, with the increased popularity of smartphones, the behavioural data presented are likely to be no longer representative of contemporary daily mobile phone activity.
Raza et al (2017) contributed further to this by highlighting that screen protectors were more likely to harbour pathogenic bacteria (62.5%; n=40) than unprotected screens (45.3%; n=29). There were no significant differences regarding screen size, duration of use at work or clinical setting.
Other factors found to increase the risk of pathogenic bacteria presence on mobile phones are a cracked screen (Qureshi et al, 2020), use of a mobile phone case (Qureshi et al, 2020; Kuriyama et al, 2021) and a lack of regular cleaning (Heyba et al, 2015; Qureshi et al, 2020).
Smartphones, along with other touchscreen devices such as tablets (which are increasingly used as electronic care records) are likely to substitute wristwatches as the primary time source for many clinicians, especially in the out-of-hospital setting. A recent X (formerly Twitter) poll found that 58.8% (n=70) of a prospective cohort of 119 respondents would use a mobile phone as an alternative time source to record a clinical assessment or intervention in an out-of-hospital environment if a watch or wall clock was not available. Within the same poll, 20.2% (n=24) of respondents would use a fob watch, and 14.3% (n=17) selected a tablet or electronic patient care record as a preferred alternative time source (Strudwick, 2023a).
All of the cited peer-reviewed studies evidence the risk of mobile phones and other touch-screen devices to patients within clinical settings—a finding that is likely to be compounded if repeated with an out-of-hospital cohort. This, however, has yet to be studied specifically.
Recommendations
Four policy evidence-based recommendations are made as follows:
- Out-of-hospital clinicians should have special dispensation within infection prevention and control (IPC) policy to wear a wristwatch when working in a patient-facing capacity following a dynamic risk assessment. This should especially apply to those working within helicopter emergency medical services (HEMS) (or similar) and hazardous area response teams (HART)
- As part of this, any wristwatch worn by a clinician should be:
- Submersible and easily cleaned
- Washed at the start and end of each shift, and additionally when visibly soiled
- Removed and washed during handwashing undertaken in accordance with the ‘Your five moments of Hand Hygiene’ framework (World Health Organization (WHO), 2009)
- Not touched during episodes of patient contact or removed following hand washing prior to patient contact
- Additional attention should be paid to ensuring that wrists are washed as part of normal hand-washing practice
- All patient-facing personnel should be aware of the risks of spreading HCAIs posed by using touch-screen devices prior to, during, and after patient contact. Doing so should be avoided unless absolutely necessary. Consideration to mitigating the risks posed by touch-screen devices is urgently required within current infection prevention and control policy.
Conclusion
This review has outlined the inadequacy of the evidence base used by NICE (2017) to inform its iteration and the enforcement of BBE policies within the out-of-hospital setting. This is equally true for the recent position statement jointly published by the Association of Ambulance Chief Executives (AACE) and the National Ambulance Service Infection Prevention and Control Group (2022), which relies upon the NICE guidance and therefore the same slim evidence base. The current review has collated a much wider collection of peer-reviewed studies relevant to BBE policy in the out-of-hospital environment and the potential knock-on effects.
This review shows wristwatches to be a potential risk of HCAI transmission but this evidence base needs focus and refinement. This risk could also be mitigated with best practice regarding regular watch-cleaning as well as effective and judicious hand- and wrist-washing techniques. This review has also hypothesised that wristwatches are no greater threat to IPC than a plain wedding band or touchscreen device, both of which have been highlighted as significant vectors for pathogen transmission but are not prohibited under current policies.
While a full BBE policy may be suitable for a conventional hospital-based care context, it must be recognised that the out-of-hospital environment presents unique challenges for care provision. The hazards of specialist care delivery in this environment, such as that by HEMS and HART, further compound these challenges. These environments necessitate the reduction of human factors in the delivery of patient care, and this policy would seem to do the opposite of this, especially in relation to the recording of manual observations and temporal data.
This has been recognised within the current Care Quality Commission (2020) inspection framework for ambulance services, and should be replicated within relevant policies. Such an approach is already being used in some services following a robust dynamic risk assessment and evidential review (Pre-hospital Care Standard Operating Procedure: Infection Prevention & Control, Essex and Herts Air Ambulance Charity, unpublished internal document, 2022). However, such an approach conflicts with the Uniforms and Workwear Guidance for NHS Employers (NHS England and NHS Improvement, 2020), which identifies the wearing of a watch as poor practice but offers no evidence to support this statement.
There is a huge appetite for this research question to be resolved, as demonstrated by the 91% (n=153) of the 168 X poll (formerly Twitter) respondents who agree that clinicians should be able to wear watches when working within out-of-hospital practice (Strudwick, 2023b). This topic area would therefore benefit from further research into the risk of wristwatches as vectors of transmission for HCAI pathogens, and also the risks posed by alternative time sources within the out-of-hospital environment. Until such time as this information becomes available, clinicians providing care in these contexts should be hyper aware of the risk their wristwatches (if they can be worn within their current policy framework) might have on IPC and do their best to mitigate these. The same is equally true for touchscreen devices and fob watches, which may be used as a substitute.
It is essential that NICE conducts a contemporary surveillance review of this topic, especially relating to IPC for out-of-hospital practice. This should include more pragmatic recommendations specifically relating to the use of wristwatches that align more closely with the peer-reviewed literature available. Ideally, this would follow the recommendations set out within the present article, which have been drafted in accordance with the material covered herein.
Key Points
- Wearing wristwatches is prohibited by bare-below-the-elbows (BBE) policies but the evidence supporting this position is slim and of variable quality
- Primarily, watches pose a barrier to cleaning the wrist. However, the risk this poses to patients is unexplored
- A poorly cleaned wristwatch is still a potential risk of HCAI transmission, especially when touched during the course of patient care, but this evidence base needs further refinement
- A wedding band, nail polish, and touch-screen devices pose a greater risk to patients as an HCAI vector than a wristwatch
- BBE policies should be reviewed to allow wristwatches to be worn within out-of-hospital care environments to mitigate risks posed by alternate time sources which need to be touched. Recommendations for this are included within this review.
CPD Reflection Questions
- What do you currently use as a time source in your practice when a clock is not available? Do you need to touch or move this in order to read it? When was it last cleaned?
- Do you consider washing your wrists and under your wedding band as part of your normal hand-washing routine?
- Have you considered the last time you cleaned the tablet computer screen and mobile phone you may use in a clinical setting?


