Asthma is a non-communicable respiratory condition which, according to the 2016 Global Burden of Disease study, affects an estimated 339.4 million people worldwide (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (GBD), 2017). While there are an estimated 1000 deaths per day globally from asthma, this study calls this the ‘tip of the iceberg’ when it comes to the overall impact of the condition (GBD, 2017). With the equivalent of an estimated 23.7 million years of full health lost to asthma globally during 2016, the morbidity of asthma puts a great burden on the daily functioning of those with the condition (Global Asthma Network, 2018).
With no definitive cure, an approach of symptom control and prevention of triggering factors has been shown to reduce morbidity and mortality (Papaioannou et al, 2015; Oland et al, 2017). The standard treatment for patients experiencing an exacerbation of their asthma has remained constant for almost 40 years and involves the use of bronchodilator drugs (beta-2 agonists) to relieve the bronchospasm associated with asthma (Sellers, 2013; Almadhoun et al, 2022).
However, in 2010, the Global Strategy for Asthma Management and Prevention was updated to suggest that there should be a move away from short-acting beta-2 agonists (SABAs) as the primarily treatment for asthma exacerbations (Global Initiative for Asthma, 2010). This has been under discussion for many years given their potential to mask ongoing exacerbations and cause adverse short- and long-term physiological changes (Billington et al, 2017; Martin and Harrison, 2019). Despite this, clinical guidelines continue to state that clinicians should ‘use high-dose inhaled beta-2 agonists as first line agents in patients with acute asthma and administer as early as possible’ (Association of Ambulance Chief Executives and Joint Royal Colleges Ambulance Liaison Committee (AACE/JRCALC), 2019; British Thoracic Society (BTS), 2019).
Although SABAs are targeted towards beta-2 adrenoreceptors responsible for the relaxation of smooth muscle, their binding ability is weak, which causes an off-target effect resulting in additional stimulation of the alpha-1, alpha-2 and beta-1 adrenoceptors (Hsu and Bajaj, 2022). More commonly seen with the high dosages associated with prehospital care (Guhan et al, 2000; Farzam et al, 2022), the resulting physiological effects can result in changes within the cardiovascular, respiratory, nervous and endocrine systems that can alter their communication pathways (Chawla et al, 2016; Billington et al, 2017; Sampson and Bersten, 2017; Hsu and Bajaj, 2022).
With such changes occurring within core systems, the implications for the presenting condition could be critical and could oppose the intended therapeutic effects of beta-2 agonists (Billington et al, 2017; Hsu and Bajaj, 2022). This may result in an extended exacerbation through perpetuation of ongoing hypoxaemia (Chawla et al, 2016; Billington et al, 2017; Sampson et al, 2017; Hsu and Bajaj, 2022) as a direct result of compounding cardiac arrhythmias (Hsu and Bajaj, 2022) and a reduction in oxygen saturations (Seifi et al, 2018).
By looking at the literature published since the Global Strategy for Asthma Management and Prevention report (Global Initiative for Asthma, 2010), this systematic review aimed to gain a better understanding of the physiological changes that occur in patients with asthma as a result of using SABAs, any characteristics of this treatment that may affect its results and any adverse effects that could potentially impact patients' short and long-term health.
Methods
Search strategy
A search of literature covering 1 January 2010 to 24 September 2020 was undertaken using PubMed, MEDLINE, CINAHL, Embase, the Cochrane Library, Web of Science, Trip and EThOS. A simplified search strategy, derived from an initial scoping review and consolidated through Medical Subject Headings (MeSH) and Embase Emtree, used the terms ‘asthma’ and ‘albuterol’ or ‘salbutamol’ or ‘Ventolin’ or ‘beta 2’. Filters were included on each database where possible, including the date range, age groups, English language and human participants. Extensive manual filtering was then undertaken to ensure all studies met the systematic review's inclusion and exclusion criteria.
The review protocol was published on PROSPERO (Registration: CRD42020200131) during September 2020. An amendment was made during November 2020 relating to the exclusion criteria.
Selection criteria
All studies that included diagnosed asthmatics treated with nebulised SABAs as part of the methods were considered. Studies had to be directly related to this review's aims by reporting on changes to the cardiac and respiratory systems and/or identifying biological characteristics that alter the mechanism of action of nebulised beta-2 agonists.
Studies were excluded from the review if patients were treated with: beta-2 agonists for conditions other than asthma (e.g. chronic obstructive pulmonary disease); long-acting beta-2 agonists (e.g. salmeterol); or other drugs were used alongside the beta-2 agonists to treat the exacerbation (e.g. hydrocortisone or ipratropium bromide). Also excluded were studies that included pregnant women, children aged under 5 years and those that involved low-dose therapy or where delivery methods other than nebulisation were used (e.g. an inhaler).
All papers meeting the inclusion and exclusion criteria were included, irrespective of methodology. Papers where only the abstract was available and systematic reviews were excluded. Final papers had their references checked to identify any eligible studies that might have been missed during the initial review.
Data extraction
Data extraction was undertaken independently by two reviewers using a modified version of the Cochrane Collaboration data collection form (Cochrane Effective Practice and Organisation of Care (EPOC), 2013). The same reviewers assessed risk of bias and quality using Cochrane Collaboration tools (EPOC, 2013; 2017) and a modified version of the Critical Appraisal Skills Programme (CASP, 2018) cohort study checklist.
Data analysis
A narrative synthesis was used to report the findings using a descriptive approach because of the heterogeneity of studies (Kim et al, 2017). Categories were derived from study characteristics and results to assist with the identification and contextualisation of specific themes (Nowell et al, 2017), offering the maximum level of comparison.
Results
Eight studies were eligible for synthesis. There was considerable variation in methods and reported outcome measures (Table 1) and often only partial datasets were relevant to the review, resulting in limited comparable data. Furthermore, following appraisal, just two studies demonstrated a low risk of bias and high level of quality (Table 2).
| Study | Location | Design | Intervention drug | Dosage/frequency | Participant group | Age | Participant numbers | Recorded outcomes | Observation time frame |
|---|---|---|---|---|---|---|---|---|---|
| Das et al (2011) | Tertiary care hospital, India | Double blind, prospective, comparative study | Nebulised salbutamol | 5 mg repeated three times (20-minute intervals over 1 hour) | Non-severe asthma patients presenting at emergency or chest outpatient department | >18 years | 25 | Peak expiratory flow rate | Baseline and 20, 40 and 60 minutes after each treatment |
| Mangunnegoro et al (2011) | Emergency department, Indonesia | Randomised, double-blind, parallel group study | Nebulised salbutamol | 2.5 mg repeated three times (20-minute intervals over 1 hour) | Emergency department patients | 15–60 years | 69 | Peak expiratory flow rate (% predicted); asthma score (Global Initiative for Asthma breathlessness criteria); arterial blood saturation (SaO2), partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) | Baseline and 20, 40, 60 at 120 minutes after each treatment |
| Hazra et al (2013) | Tertiary care centre, India | Double-blind, prospective, comparative study | Nebulised salbutamol | 5mg repeated three times (30-minute intervals over 90 minutes) | Pulmonary medicine outpatients | >18 years | 20 | Peak expiratory flow rate; oxygen saturation—blood (SaO2) | Baseline and 30, 60 and 90 minutes after each treatment |
| Sahan et al (2013) | Emergency department, location not stated | Cross-sectional study | Nebulised salbutamol | 5mg repeated three times (20-minute intervals over 1 hour) | Emergency department patients | 35–74 years | 26 | Potassium levels | Baseline and after overall treatment (60 minutes) |
| Barkiya et al (2016) | Paediatric emergency department, India | Randomised, parallel group study (unclear if blinded) | Nebulised salbutamol | 2.5 mg repeated three times (20-minute intervals over one hour) | Patients presenting with acute exacerbation of asthma | 5–15 years | 30 | Heart rate, respiratory rate, oxygen saturation—peripheral (SpO2); forced expiratory volume; asthma score (Pulmonary Index Score) and serum potassium | Baseline and after overall treatment (60 minutes) |
| Lorensia et al (2016) | Hospitals, Indonesia | Cross-sectional study | Nebulised salbutamol | Not stated | Patients in hospital with asthma exacerbation | ≥18 years (reports ≥17) | 21 | Sodium/potassium levels | Baseline and after overall treatment (60 minutes) |
| Nabi et al (2017) | Tertiary care hospital, India | Single-blind, prospective, randomised, parallel group study | Nebulised salbutamol | 5 mg repeated three times (20-minute intervals over 1 hour) | Patients at emergency department or chest medicine outpatients clinic | >18 years | 30 | Peak expiratory flow rate | Baseline and 20, 40 and 60 minutes after each treatment |
| Ozer et al (2018) | Children's hospital, Turkey | Cross-sectional study | Nebulised salbutamol | 0.15 mg/kg repeated three times (20-minute intervals over 1 hour) | Paediatric allergy clinic or paediatric emergency department patients with acute asthma | 6–18 years | 6–11 years:123; 12–18 years: 38 | Oxygen saturation: peripheral (SpO2); asthma score (Modified Pulmonary Index Score); SpO2 defined hypoxia | Baseline and 20, 40 and 60 minutes after each treatment |
| Study | Risk of bias | Quality assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Performance | Detection | Attrition | Reporting | Validity | Results | Implications | Total score | |
| Ozer et al (2018) | Low | High | High | High | Unclear | 9 | 2 | 5 | 16 |
| Nabi et al (2017) | Low | *Unclear | Low | High | High | 10 | 3 | 5 | 18 |
| Barkiya et al (2016) | Low | *Unclear | Low | Low | Unclear | 14 | 6 | 6 | 26 |
| Das et al (2011) | Low | *Unclear | Low | High | High | 10 | 3 | 5 | 18 |
| Lorensia et al (2016) | *Unclear | *Unclear | Low | High | High | 3 | 2 | 3 | 9 |
| Hazra et al (2013) | Low | *Unclear | High | Low | Low | 10 | 4 | 5 | 19 |
| Sahan et al (2013) | Low | *Unclear | High | Unclear | Unclear | 12 | 4 | 6 | 22 |
| Mangunnegoro et al (2011) | Low | Low | Low | Low | Low | 16 | 6 | 6 | 28 |
The PRISMA methodology (Page et al, 2020) was used to structure the search process for this review and is presented in Figure 1.
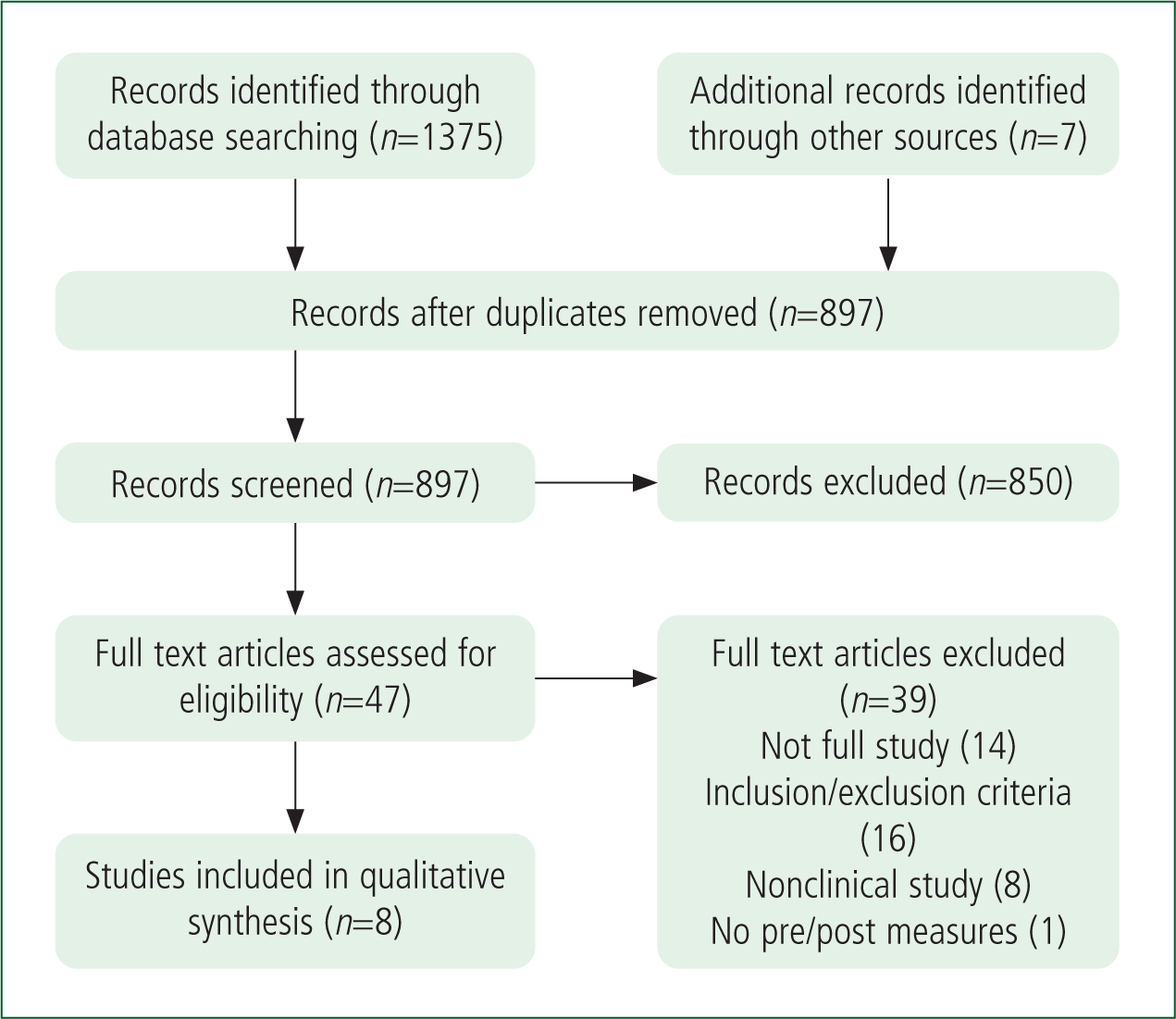
Demographics
Of the eight studies, only Mangunnegoro et al (2011), Barkiya et al (2016) and Ozer et al (2018) included children (aged <18 years). The other five studies focused on adult populations although it is unclear in the Lorensia et al (2016) study whether their youngest participants were aged 17 or 18 years. Only Ozer et al's (2018) study divided the sample into age groups. It was therefore not possible to use age as a comparable variable.
Drug administration
All studies administered salbutamol rather than alternative SABAs.
Though three studies did not explicitly state how this was done (Sahan et al, 2013; Barkiya et al 2016; Lorensia et al, 2016), it is expected that seven of the studies used oxygen-driven nebulisers given their setting, while Mangunnegoro et al (2011) used a compressor nebuliser.
All adult-only studies used a repeat dose of 5 mg with those involving children using a lower dose of 2.5 mg or a weight-based dose of 0.15 mg/kg. Lorensia et al (2016) did not identify the dose given.
In six studies, the drug was administered in 20-minute increments, Hazra et al (2013) used 30-minute increments and Lorensia et al (2016) did not specify any timings.
Clinical observations
In all studies, measurements were undertaken at baseline and at least one other time point after drug administration, either after each dose or after the final dose. The observations and results are shown in Table 3.
| Authors | Recorded outcomes | Results | Side effects stated | Comorbidities noted |
|---|---|---|---|---|
| Das et al (2011) | Peak expiratory flow rate | Peak expiratory flow rate showed significant increase over the baseline values and the increase was evident after each dose of the drug | Headache: 2 |
No |
| Mangunnegoro et al (2011) | Peak expiratory flow rate (% predicted); asthma score (Global Initiative for Asthma breathlessness criteria); arterial blood saturation (SaO2), partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) | Significant improvements in peak expiratory flow rate and asthma score were seen. A slight reduction (95.3%–94.3%) in mean SaO2 was shown between base and 120 minutes. PaO2 and PaCO2 also showed a slight decrease at 120 minutes. Tachycardia seen within ECG traces at 120 minutes | Palpitations: 2 |
Hyperinflation Hypervascular pneumonia Pulmonary tuberculois and post tuberculois Bronchiectasis Diabetic patients excluded |
| Hazra et al (2013) | Peak expiratory flow rate; oxygen saturation—blood (SaO2) | Peak expiratory flow rate was significantly higher than at baseline, with increases after each dose. Increases in pulse and respiratory rates was seen, with oxygen saturation almost within the normal range | Headache: 1 |
No |
| Sahan et al (2013) | Potassium levels | Serum potassium levels fell significantly by 0.3 mg/dl (from 4.6 mEq/l to 4.3 mEq/l; P≤0.001]). Serum phosphate levels fell by 0.1 mg/dl, demonstrating no significant decrease | No | No |
| Barkiya et al (2016) | Heart rate, respiratory rate, oxygen saturation—peripheral (SpO2); FEV1, asthma score (Clinical Asthma Severity Score) and serum potassium | Mean SpO2 improved between baseline and 60 minutes (94.76% to 97.93%). A clinical improvement in FEV1 and PIS, plus a fall in respiratory rate from 28.20 bpm to 22.6 bpm were seen. Significant tachycardia (mean increase from 101.8 bpm to 124.6 bpm) and a fall in potassium levels (from 4.26 mEq/l to 3.64 mEq/l were seen at 0.2–0.6 mEq/l from baseline (P=0.0001) | No | No |
| Lorensia et al (2016) | Sodium/potassium levels | A fall in potassium levels was seen in 13 (30.23%) participants, with five (11.63%) remaining the same and three (6.98%) seeing an increase. A decrease in sodium levels was seen within six (13.95%), nine (20.93%) had no change and six (13.95%) saw a rise. Hypokalaemia was experienced by 11.63% of the subjects | No | Gastritis Type 2 diabetes Dyslipidaemia |
| Nabi et al (2017) | Peak expiratory flow rate | Peak expiratory flow rate showed significant increase over baseline values and the rise was evident after each dose of the drug | Headache: 2 |
No |
| Ozer et al (2018) | Oxygen saturation: peripheral (SpO2); asthma score (Modified Pulmonary Index Score); SpO2 defined hypoxia | Salbutamol-induced hypoxia was 23.7% at 12–18 years and 13% at 6–11 years after initial salbutamol dose, with SpO2 levels up to 92%. MPIS reduced over three doses in all age groups | No | Atopic dermatitis |
bpm: beats per minutes; ECG: electrocardiogram; FEV1: forced expiratory volume in 1 second; MPIS: modified pulmonary index score PIS: pulmonary index score
Blood oxygenation
Ozer et al (2018) and Barkiya et al (2016) recorded peripheral capillary oxygen saturation levels using a pulse oximeter (SpO2) and Mangunnegoro et al (2011) and Hazra et al (2013) used blood samples to gain arterial oxygen saturation levels (SaO2).
Both Ozer et al (2018) and Barkiya et al (2016) demonstrated a trend towards an improvement in SpO2 during treatment, although Ozer et al (2018) reported salbutamol-induced hypoxia following the initial dose. Hazra et al (2013) used SaO2 and just stated that ‘oxygen saturation was almost within normal ranges’ without further clarification. Mangunnegoro et al (2011) demonstrated a slight reduction in mean SaO2 between baseline and 120 minutes.
Lung function
Das et al (2011), Mangunnegoro et al (2011), Hazra et al (2013) and Nabi et al (2017) measured peak expiratory flow rate and Barkiya et al (2016) forced expiratory volume. All studies suggested lung function improved by increases from baseline following each dose.
Heart rate and respiratory rate
Baseline and post treatment heart rate (HR) and respiratory rate (RR) figures were reported only by Barkiya et al (2016), who found an improvement in RR and the presence of a significant tachycardia after all doses. Ozer et al (2018) and Mangunnegoro et al (2011) both recorded HR and RR for use as part of their scoring system, with Mangunnegoro et al (2011) reporting the presence of tachycardia within electrocardiogram traces at 120 minutes. The Hazra et al (2013) study did state that an increase in pulse and RR was seen, although no figures were provided.
Asthma scoring
Mangunnegoro et al (2011), Barkiya et al (2016) and Ozer et al (2018) all used scoring tools to assess the severity of the presenting exacerbation at baseline and after treatment.
Though these tools differed, they shared common variables: Barkiya et al (2016) used the Clinical Asthma Severity Score (which includes RR, oxygen saturations, wheeze, retractions and dyspnoea); Ozer et al (2018) the Modified Pulmonary Index Score (HR, RR, oxygen saturations, wheeze, accessory muscle use and inhalation-exhalation ratio); and Mangunnegoro et al (2011) used a tool based on the Global Initiative for Asthma 1998 criteria of breathlessness (HR, RR, wheezing, breathlessness, talking, alertness, accessory muscles and suprasternal retractions and peak expiratory flow rate).
All three studies demonstrated a reduction in severity from baseline, suggesting nebulised salbutamol reduces the severity of an asthma exacerbation.
Sodium and potassium levels
Sahan et al (2013), Barkiya et al (2016) and Lorensia et al (2016) recorded serum potassium levels, with Lorensia et al (2016) also measuring sodium levels.
All three studies showed a decrease in potassium levels in the majority of patients with Barkiya et al (2016) demonstrating a significant decrease (0.5-0.8 mEq/l from baseline values) in 70% of participants following nebulisation. Lorensia et al (2016) reported an inconclusive range of changes in sodium levels. These data strongly suggest a correlation between the administration of salbutamol and reduction in serum potassium, potentially to a level of hypokalaemia.
Patient-reported side effects
Half of the studies identified patient-reported side effects (Das et al, 2011; Mangunnegoro et al, 2011; Hazra et al, 2013; Nabi et al, 2017). These included headaches, tremors, palpitations and nausea/oral irritation. Although the results were not definitive, the numbers indicated that at least 10% of those receiving repeated doses of 5 mg salbutamol experienced some form of patient-identifiable side effect; where lower doses were used, this frequency was lower.
Comorbidities
Mangunnegoro et al (2011), Lorensia et al (2016) and Ozer et al (2018) specifically identified participant comorbidities.
Mangunnegoro et al (2011) excluded patients with diabetes and Ozer et al (2018) mentioned conditions that predominantly present alongside asthma as part of the immunoglobulin E (IgE) sensitisation pathway.
Lorensia et al (2016) identified one participant with diabetes, one with raised cholesterol and one with gastritis. The patient with diabetes demonstrated hypokalaemia following treatment but, as this was only a single study that involved three participants with conditions that sit outside the IgE pathway, no relationship can be identified between diabetes and potassium levels.
Discussion
Recent years have seen significant discussion around how best to manage asthma exacerbations and there is debate on the appropriateness of using SABAs. However, guidelines developed for UK ambulance services continue to support their repeated use as the first pharmacological step in managing these acute events (AACE/JRCALC, 2019; BTS, 2019).
Characteristics
Key characteristics for review in this study were dosage, age and recorded outcomes. The age demographic is important given the pathogenesis of asthma and how patients seem to outgrow the condition or develop it in later life (Warke et al, 2002; Trivedi et al, 2019).
Only patients aged ≥5 years were included within the review but investigation of age as a variable was not possible as only one study subdivided participants by age. Paediatric patients did receive a lower dose than adults and studies that monitored clinical observations throughout the treatment time frame demonstrated the effects of a cumulative dose upon cardiovascular and respiratory observations in line with evidence produced by previous studies (Sellers, 2013).
Only a general conclusion can be made from this review of these study characteristics and that is that most asthma patients aged ≥5 years respond similarly to the administration of nebulised salbutamol when aligned with the dosing recommendations of the National Institute for Health and Care Excellence (NICE, 2021).
Changes in clinical observations
Studies using lung function testing and asthma scoring as indicators of the effectiveness of salbutamol reported improvements through the course of treatment. This supports the rationale for administering SABAs to asthmatics experiencing an exacerbation, but does not contribute to the overall understanding of the drug.
Current guidelines suggest that arrhythmias, especially tachycardia, are frequent side effects seen in patients receiving SABAs (AACE/JRCALC, 2019; BTS, 2019; NICE, 2021). Despite this, several studies failed to record or report the HR, suggesting that its importance in the overall rating of an exacerbation is often underestimated (Rolfe, 2019; Sapra et al, 2022). While only three studies reported this parameter, all noted an increased HR, supporting previous incidences where this has been recorded (Sears, 2002; Cazzola et al, 2013).
Disruption to the cardiovascular and respiratory systems can result in a lung ventilation/perfusion ratio (V/Q) mismatch (Petersson et al, 2014; Sarkar et al, 2017) and the result of this is seen in a reduction of blood oxygenation levels.
While SaO2 is generally regarded as the gold standard for assessing oxygenation levels (Bilan et al, 2010; Chan et al, 2013), the convenience of SpO2 monitoring makes it the first choice for ongoing patient assessment in the prehospital setting. However, the use of SpO2 to provide definitive values has been questioned, given its potential to underestimate or overestimate readings (Muñoz et al, 2008; Chan et al, 2013; Amalakanti et al, 2016) as a result of poor peripheral circulation or external factors such as skin temperature and movement (Bilan et al, 2010; Guler et al, 2016).
The selected studies showed the presence of reduced blood oxygenation levels and do suggest an increase follows drug administration over a 60-minute time frame. Some individuals may demonstrate a reduced level of oxygenation following initial treatment for reasons not explained, further indicating that oxygen levels are transient depending upon age and severity of exacerbation (Gleeson et al, 1988).
Furthermore, the level of V/Q mismatch could be such that a correlation between SaO2 and SpO2 may not be instantaneous, given the reactive mechanisms during hypoxia that result in peripheral shutdown of the circulatory system to prioritise core tissue such as the heart and brain (Chan et al, 2013; Seifi et al, 2018).
In line with current literature and guidelines, the studies show that both favourable and adverse effects are most commonly seen within the cardiac and respiratory systems with a clear understanding of their cause (Hsu and Bajaj, 2022). However, other effects reported by the studies are less obvious and may impact the patient's condition in more varied ways.
The decrease in serum potassium levels (Sahan et al, 2013; Barkiya et al 2016; Lorensia et al, 2016) has been widely reported in past studies (Heianza et al, 2011) and this mechanism may be used therapeutically for patients presenting with hyperkalaemia (Viera and Wouk, 2015).
Although potassium is key to ensuring electrical functions such as muscle stimulation and nerve impulses are maintained within suitable limits (Udensi and Tchounwou, 2017), its change in the short term is not usually problematic. However, when levels reach that of hypokalaemia and are present in the longer term, wider functions including those within the respiratory, cardiovascular, endocrine and nervous systems may be affected (Udensi and Tchounwou, 2017). This trigger, secondary to that of receptor stimulation, can result in the more commonly seen adverse effects of beta-2 agonist administration, such as arrhythmias, palpitations, headache, muscle twitching, malaise and subdued insulin production and tolerance (Heianza et al, 2011; Petersson et al, 2014; Udensi and Tchounwou, 2017; NICE, 2021).
With these cardiac and electrolyte changes, the potential for asthma patients already experiencing a level of V/Q mismatch to be impacted to a greater degree is high (Sarkar et al, 2017). It may also explain why caution may be required when administering beta-2 agonists to patients with diabetes, given the potential for blood glucose levels to rise as a result of retarded insulin production (Jacques et al, 2013). This is something one study alluded to when a diabetic participant presented with hypokalaemia following treatment, although, given the high bias and low-quality assessment in this study, the potential correlation between diabetes and potassium levels cannot be corroborated.
Therefore, while the presented data continue to support the therapeutic benefits of SABAs in the management of asthma exacerbations, it is also clear that changes to HR and electrolyte levels accompany these. However, despite both having the potential to affect patients whether they have underlying medical conditions or not (Sevransky, 2009; Udensi and Tchounwou, 2017), none of the included studies reported on whether the adverse effects of beta-2 agonists impacted the patients' underlying health conditions.
This leaves a gap in our understanding given the range of potentially compounding health conditions that may be present. Future studies need to investigate these potential associations and the significance of any links identified.
Implications for practice
This review highlights the limited data available on which to base any definitive conclusions about the use of beta-2 agonists in the treatment of asthma exacerbations. However, there is sufficient evidence to suggest that some patients may experience potentially significant adverse effects that are not commonly identified, considered or reported.
Further investigation of the full effects of nebulised beta-2 agonists and how different patients react to the drug during an acute exacerbation will help optimise future treatment for the asthmatic population. Any actionable findings could assist with limiting adverse effects, potentially reducing hypoxaemia, hypokalaemia and exacerbation times.
Limitations
The inclusion and exclusion criteria used in this review resulted in a small heterogeneous sample of articles being selected and, at times, in partial data sets being abstracted, thereby reducing the scope for comparison between studies.
Of the data sets identified, there were inconsistencies that further decreased the level of comparison and correlation that could take place. This can be seen where, despite the subject matter, a number of studies did not report on basic clinical observations such as HR and RR, both of which are crucial markers when assessing the severity of an exacerbation (Aldington, 2007).
Even when clinical values were assessed across multiple studies, a difference in testing techniques again reduced the overall level of comparability. For example, there were differences in how blood oxygenation was measured.
Additionally, the overall quality and risk of bias in the majority of the studies has resulted in a lower confidence in the true value of any outcomes. It is, however, accepted that while the Cochrane risk of bias tool was used, the additional CASP (2018) appraisal tool, although widely established, was modified to include a numerical rating system. Although this did not alter the focus of the tool, it may reduce its validity within this review.
Conclusion
This systematic review supports the use of SABAs in patients experiencing an uncontrolled exacerbation of their condition.
A clear post-treatment reduction in exacerbation severity and improvement in overall lung function has been shown throughout.
It has not been possible to establish the level, frequency and range of adverse effects, although the non-selective nature of beta-2 agonists can be seen within the functionality of both the cardiovascular and endocrine systems.
Despite asthma being associated with non-related comorbidities, it is unclear how these conditions may be affected by any of the adverse changes identified, although variations in HR, electrolytes and oxygen saturation levels can all impair normal physiological functions.
Additional research is required to understand the complexities of the systemic and non-systemic changes experienced by asthma patients receiving nebulised SABAs, with a focus on how these manifest and impact upon underlying conditions.
LEARNING OUTCOMES
After completing this module, the paramedic will be able to:

