Cardiovascular diseases are the leading cause of death globally, responsible for 17.9 million annual deaths, which represent 32% of all global mortality (World Health Organization (WHO), 2021). Acute coronary syndrome (ACS) accounts for nearly half of these deaths and contributes to 12% of disability-adjusted life years lost (Tsao et al, 2022). Beyond the significant human cost, ACS imposes a substantial economic burden. In the UK, cardiovascular diseases cost approximately £9 billion annually, with ACS accounting for 75% (Zaki et al, 2023). Approximately 2.3 million individuals in the UK are affected by ACS, contributing to 66 000 deaths annually, including 25 000 occurring in those under 75 years. Effective ACS management at all stages – prehospital, hospitalisation, and post-discharge – significantly improves outcomes. Evidence suggests that all three phases are equally critical, as both short- and long-term ACS outcomes depend on the quality of care at each stage (Tzabo et al, 2021). Therefore, paramedics play a vital role in the early recognition and management of ACS, ensuring timely treatment, appropriate decision-making, and optimal place of care for each patient. To understand the importance of early and effective pharmacological management, it is key to explore the underlying pathophysiology of ACS and its progression from coronary artery disease.
Pathophysiology
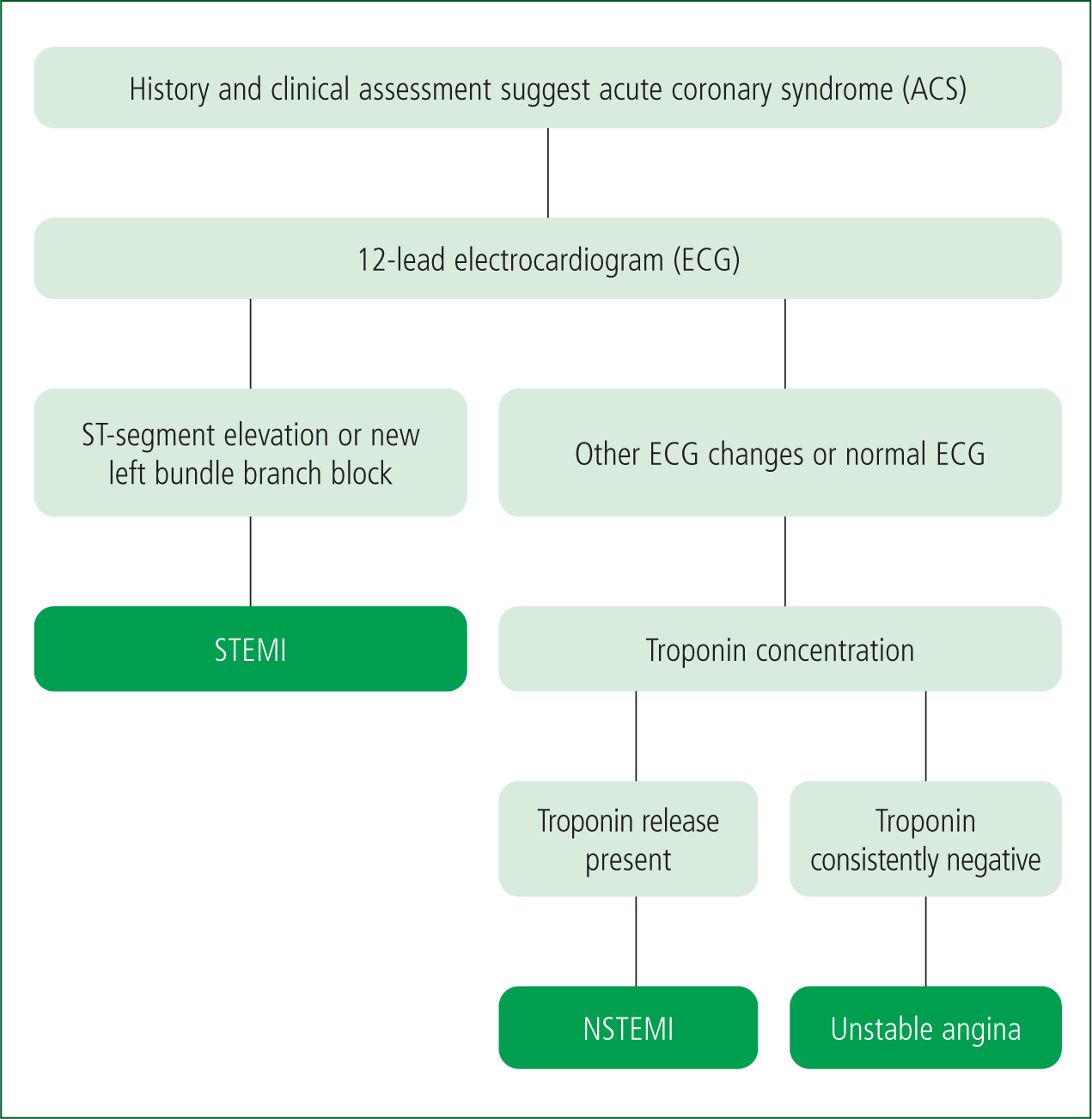
ACS is a group of clinical conditions resulting from acute myocardial ischaemia, primarily owing to a sudden reduction in coronary perfusion, leading to decreased oxygen delivery and subsequent myocardial damage. It encompasses the following three main conditions classified based on electrocardiogram (ECG) findings and biomarkers of myocardial injury (Figure 1) (Nikolaou et al, 2015):
The most common underlying cause of ACS is coronary artery disease – a progressive condition in which fatty deposits (atheroma) accumulate in the walls of the coronary arteries, leading to a narrowing and reduction in the elasticity of the vessels (Smit and Lochner, 2020). While other causes, such as calcification, coronary vasospasm, and coronary dissection, can contribute to coronary artery disease, atherosclerosis is the predominant mechanism (Falk, 2006).
The development of atherosclerosis occurs in several stages. The process begins when factors such as smoking damage the vessel walls, often followed by physiological changes such as high blood pressure, which arise as a result. Once the vessel walls are compromised, cholesterol can enter and trigger inflammation. Over time, fibrous plaques may form; however, continued inflammation makes these plaques prone to rupture. Plaque expansion can partially occlude coronary arteries, reducing blood flow and causing ischaemia, which may present as angina.
If a plaque ruptures, its contents enter the bloodstream, leading to platelet adhesion, aggregation, and activation of the coagulation cascade. This results in thrombus formation, which may partially or completely obstruct arterial blood flow, causing ischaemia or infarction of the heart muscle, clinically manifesting as ACS (Falk, 2006).
Pharmacological management
The initial pharmacological management of ACS aims to alleviate ischaemic pain, reduce myocardial oxygen demand, halt further thrombus formation, and prevent progression of cardiac injury (Joint Formulary Committee, 2025). In the prehospital setting, first-line pharmacological interventions include aspirin, glyceryl trinitrate (GTN), and morphine (JRCALC, 2022; Joint Formulary Committee, 2025). Additional pharmacological options – including dual antiplatelet therapy (DAPT) and fibrinolytic therapy – are administered based on the patient's clinical presentation, the availability of primary percutaneous coronary intervention (PPCI), and legal frameworks and guidelines (England, 2016; National Institute for Health and Care Excellence (NICE), 2020). This section reviews the mechanism of action, clinical benefit, and evidence base for these interventions.
Aspirin
Aspirin is a fundamental component of ACS management owing to its well established antiplatelet effects, which reduce thrombus formation and arterial occlusion. It is typically administered orally, in the form of tablets, chewable tablets or powder. After oral administration, it is absorbed in the stomach and small intestine, then converted in the liver to salicylic acid, the active metabolite. Aspirin exerts its mechanism of action through irreversible acetylation of cyclooxygenase-1 (COX-1), thereby blocking the conversion of arachidonic acid into prostaglandins. This inhibition prevents the formation of thromboxane A2 (TXA2) – a key mediator of platelet aggregation and vasoconstriction – thereby reducing thrombus formation and arterial occlusion (Angiolillo, 2012). By reducing platelet aggregation, aspirin contributes to the maintenance of coronary artery patency and decreases the risk of myocardial infarction (MI), stroke, and pulmonary embolism. Early administration of aspirin in ACS has been consistently linked with improved short- and long-term survival outcomes (Barbash et al, 2002; Djarv et al, 2020). Evidence suggests that aspirin is most effective when administered within 2 hours of symptom onset, although the precise time window for maximal benefit remains uncertain (Djarv et al, 2020). Given its favourable risk-benefit profile, aspirin should be administered promptly upon ACS diagnosis, unless contraindicated.
P2Y12 adenosine diphosphate antagonists
The P2Y12 adenosine diphosphate (ADP) antagonists are used to inhibit platelet activation by blocking P2Y12 ADP receptors on platelets – the main receptor responsible for mediation of ADP-induced platelet aggregation (Fabris et al, 2021). These include clopidogrel, prasugrel, and ticagrelor, which can be used alongside aspirin as a DAPT. DAPT is particularly indicated for patients with ACS undergoing PPCI; it significantly reduces the risk of stent thrombosis and recurrent MI and should be offered to most patients with STEMI (Joint Formulary Committee, 2025). The choice of P2Y12 ADP antagonist depends on the planned intervention – PPCI, fibrinolysis, or conservative management – as well as the patient's bleeding risk (Joint Formulary Committee, 2025).
Prasugrel is the preferred agent for most patients undergoing PPCI, unless the risk of bleeding outweighs its benefits. Ticagrelor serves as an alternative because of its rapid onset of action, proven efficacy in reducing major adverse cardiac events, and the fact that it reversibly inhibits platelet aggregation, allowing for faster recovery if needed. Unlike clopidogrel, it does not require metabolic activation and has a more predictable effect. Clopidogrel is typically reserved for cases where prasugrel or ticagrelor are contraindicated, particularly in patients with high bleeding risk or those receiving fibrinolytic therapy. In patients not undergoing PPCI, especially those with a high risk of bleeding, aspirin monotherapy may be the safest option to minimise haemorrhagic complications (Joint Formulary Committee, 2025).
Prehospital administration of clopidogrel has been associated with a reduction in major adverse coronary events; however, clinical trials have not demonstrated a significant impact on myocardial reperfusion (Fabris et al, 2017). The ATLANTIC trial assessed prehospital ticagrelor administration, concluding that while it was safe and did not increase bleeding risk, its antiplatelet effects remained limited before PPCI (Fabris et al, 2017). Nonetheless, early administration may be advantageous in cases where PPCI is delayed, allowing for earlier platelet inhibition and thrombus stabilisation (Fabris et al, 2017).
Glyceryl trinitrate
GTN is an effective intervention for the management of ischaemic chest pain in ACS and should be considered unless contraindicated by hypotension, bradycardia, or inferior infarction with suspected right ventricular involvement (Nikolaou et al, 2015). As a potent vasodilator, GTN acts by binding to vascular smooth-muscle receptors, stimulating nitric oxide (NO) production and increasing cyclic guanosine monophosphate (cGMP) levels. This process inhibits calcium influx, inducing smooth-muscle relaxation, and results in vasodilation (electronic Medicines Compendium (eMC), 2023). While GTN is effective at dilating coronary arteries, its effect may be limited in vessels that are heavily occluded or structurally compromised, which is often seen in ACS (Divakaran and Loscalzo, 2017). A common misconception is that GTN directly dilates the coronary arteries. Its primary action is to reduce preload via peripheral venous dilation. However, evidence shows that GTN can improve myocardial perfusion by dilating collateral vessels – small alternative blood pathways that bypass occluded arteries – offering an alternative route for blood flow (Divakaran and Loscalzo, 2017). Some dilation of epicardial coronary arteries may also occur, but to a lesser extent.
The primary haemodynamic effects of GTN include reducing myocardial workload and improving oxygen delivery through preload and afterload modulation. Preload refers to ventricular filling pressure, influenced by venous return, while afterload denotes the resistance the heart must overcome to eject blood, primarily determined by arterial pressure and vascular tone. GTN reduces preload by dilating venous capacitance vessels, thereby decreasing ventricular filling and myocardial oxygen demand. Simultaneously, arterial dilation lowers afterload, reducing systemic vascular resistance and improving myocardial oxygen supply-demand balance. These combined effects alleviate myocardial strain and can help to relieve symptoms of ACS.
Guidelines recommend that GTN be used with caution in patients with compromised cardiac output, including those with hypotension, bradycardia, or inferior infarction with suspected right ventricular involvement. In these patients, its use may lead to a further decrease in blood pressure and cardiac output, potentially worsening haemodynamic instability to detrimental effect (JRCALC, 2022). However, evidence suggests that GTN does not significantly increase relative risk; this highlights the need to carefully balance its therapeutic benefits against the potential for transient hypotension (Robichaud et al, 2016; Wilkinson-Stokes et al, 2022).
Prehospital administration of GTN and aspirin in suspected ACS has been shown to be beneficial with minimal risk of adverse events (Nakayama et al, 2022). While current guidelines do not specify the preferred order of administration, some evidence suggests that giving aspirin before GTN (within 5–10 minutes) may reduce pain, lower opioid requirements, and decrease the need for additional GTN doses, possibly by preventing thrombus propagation and re-occlusion (Todoroski, 2021). However, guidelines recommend the prompt administration of GTN for pain relief in ACS (Joint Formulary Committee, 2025).
Morphine
Pain triggers an increased sympathetic nervous system response, leading to vasoconstriction and elevated cardiac workload. Therefore, adequate pain relief is essential, not only for patient comfort, but also to mitigate physiological stress on the cardiovascular system (O'Gara et al, 2012). Intravenous morphine is indicated for ACS pain unrelieved by GTN (Joint Formulary Committee, 2025; JRCALC, 2022). As an opioid analgesic, morphine binds to mu (μ) and kappa (κ) opioid receptors, inhibiting pain transmission in the spinal cord and central nervous system. While it provides effective analgesia and anxiolysis, activation of μ-opioid receptors can also cause hypotension, bradycardia, and respiratory depression. Careful titration is necessary to minimise respiratory depression and haemodynamic instability. Morphine also triggers the release of histamine from mast cells, which can cause side effects like itching, hypotension, and bronchoconstriction. These effects result from the role of histamine role in vasodilation and other physiological responses.
Additionally, morphine has been shown to reduce the bioavailability of P2Y12 ADP inhibitors, thereby decreasing their antiplatelet effect (Kubica et al, 2016; Adamski et al, 2017; Ostrowska and Gorog, 2020). While observational studies link morphine to increased mortality and reinfarction rates, no significant adverse outcomes in patients with STEMI undergoing PPCI have been observed (Batchelor et al, 2020). Given these considerations, morphine should be titrated to the lowest effective dose to balance analgesia with haemodynamic stability (Kubica et al, 2022). Non-steroidal anti-inflammatory drugs (NSAIDS) should be avoided owing to prothrombotic effects (Kearney et al, 2006).
Primary percutaneous coronary intervention and fibrinolytic therapy
PPCI is the preferred reperfusion strategy for STEMI when available within the recommended 120 minutes (NICE, 2020). If timely PPCI is not feasible, fibrinolysis remains a viable alternative. Fibrinolytic agents, including streptokinase, alteplase, tenecteplase, and reteplase, work by dissolving blood clots that have formed in blood vessels. Their mechanism of action involves the activation of the body's natural fibrinolytic system. They activate plasminogen, a protein that is normally present in the blood and bound to fibrin (i.e. the protein that forms the structural framework of clots). Once activated, plasminogen is converted to plasmin, which is the enzyme responsible for breaking down fibrin. Plasmin breaks down fibrin in the clot by cleaving it into smaller fragments. This process is known as fibrinolysis, which dissolves the clot and restores normal blood flow in the affected vessel (Alsomali et al, 2024). Of these, tenecteplase is generally preferred because of its lower risk of non-cerebral bleeding (Van de Werf, 1999). While fibrinolytic therapy improves survival, PPCI has superior outcomes, reducing mortality and reinfarction rates. Fibrinolysis is associated with an increased risk of major adverse cardiac events, bleeding, and reinfarction compared with PPCI (Grines et al, 2003). Therefore, PPCI remains the gold standard (Alsomali et al, 2024).
Other considerations
Nausea and vomiting are common in ACS, often worsened by opioid analgesia. Antiemetics, such as ondansetron and metoclopramide, can improve patient comfort and reduce aspiration risk (NICE, 2025a). Ondansetron acts as an antagonist to 5-hydroxytryptamine type 3 (5-HT3) receptors, preventing nausea triggered by serotonin release. Ondansetron has been found to prolong the QT interval, increasing the risk of arrhythmias such as torsades de pointes, particularly in patients with cardiovascular disease including ACS (Hafermann et al, 2011; Singh et al, 2023). Metoclopramide antagonises both dopamine D2 and 5-HT3 receptors, reducing nausea while also enhancing gastric emptying by increasing stomach muscle tone. As a 5-HT3 antagonist, there are concerns over the adverse cardiovascular events associated with metoclopramide, including prolonged QT interval, but these are reported to be rare (Rumore, 2012).
Oxygen saturation should be monitored in all ACS cases, with supplemental oxygen administered only when indicated (NICE, 2025b). Oxygen should be administered initially to reach a normal or near-normal oxygen-saturation level. In most acutely ill patients with normal or low arterial carbon dioxide (PaCO2) levels, the target oxygen saturation should be between 94% and 98% (NICE, 2025b). While historically given to all acute patients with MI, routine oxygen therapy in normoxemic individuals does not improve mortality (Hofmann et al, 2014) and may increase myocardial injury (Stub et al, 2015; Kojima et al, 2022).
Reflective activity
Now consider the following reflective activity and the associated CPD reflection questions included in the box below:
You are called to a 58-year-old male experiencing central chest pain at approximately 5:00 am. On arrival, the patient is clutching his chest. He is alert but anxious, diaphoretic, pale and visibly distressed. He tells you he was awoken from sleep 1 hour ago by the pain, which has gradually worsened since. He has a past medical history of hypertension, hyperlipidaemia and angina and is medicated for these. He is a smoker (20 a day) and has a family history of heart disease. He tells you his pain is a 9/10 and describes the pain as ‘crushing’ and radiating down his left arm. His observations are: heart rate (HR) 112 beats per minute; blood pressure (BP) 160/94 mmHg; respiratory rate (RR) 22 breaths per minute; oxygen saturations (SpO2) 94%; temperature 35.9°C; blood glucose 8.1 mmol/l.
His ECG shows 3 mm of ST elevation in V2 and V3 and 2 mm of ST elevation in V4. You diagnose him with a STEMI.
(See questions in CPD Reflection Questions box below to complete this section).
Conclusion
The prehospital management of ACS has an impact on improving patient outcomes and reducing mortality. Paramedics play a central role in the early recognition, pharmacological intervention, and risk-stratification of patients with ACS, ensuring timely treatment and optimal care pathways. Pharmacological strategies, including aspirin, GTN, and morphine, form the cornerstone of early ACS management. DAPT and fibrinolysis provide additional options depending on guidelines and local variations, particularly when PPCI is unavailable.
While pharmacological interventions are essential, they must be carefully balanced against potential adverse effects. Opioid analgesia, despite its efficacy, may impair platelet inhibition, while antiemetics have been associated with cardiovascular adverse events in patients with ACS. Additionally, routine oxygen therapy in normoxemic individuals should be avoided. Paramedics have an important role in optimising ACS management, reducing complications and enhancing long-term outcomes.
Key Points
CPD Reflection Questions
Pertaining to the reflective activity in the text, consider the following questions:

