Stroke is a neurological emergency that results from the disruption of blood supply to part of the brain, either by infarction or haemorrhage. In the UK alone, there are more than 100 000 strokes each year; its mortality is signified by its place as the fourth biggest killer nationally (King's College London, 2018). Despite many advances in management, acute ischaemic stroke remains a leading cause of death and disability in the UK. With the cost to society estimated at £26 billion per year, medical research has focused on improving the initial recognition and treatment of these patients (Patel et al, 2017). In the mid 1990s, the importance of prehospital stroke identification accelerated with the introduction of intravenous tissue-type plasminogen activator (tPA) as a successful management strategy for ischaemic stroke (Cameron et al, 2015). These drugs are effective only if administered rapidly after the onset of stroke symptoms (Cameron et al, 2015).
To improve response times and outcomes, a systematic change that involved the whole integrated emergency response network was required (Cameron et al, 2015). These changes included:
In the late 1990s and early 2000s, stroke services were reorganised in the UK and worldwide to improve speed and access (Cameron et al, 2015). Patients are no longer routinely transported to their local emergency department; instead, they are rapidly conveyed to a stroke centre where scanning and tPA can be provided (Cameron et al, 2015).
Across the UK, most ambulance services use the Face Arm Speech Test (FAST) in the identification of stroke. While this tool has high levels of accuracy, it is recognised to be limited in its ability to diagnose all types of stroke, particularly those involving the posterior circulation (Fothergill et al, 2013). Recognition of stroke is the pivotal first step in stroke care and various stroke recognition tools are available. However, analysis of the effectiveness of these tools is beyond the scope of this article, which will focus on differentiation of stroke, with a particular emphasis on large vessel occlusion (LVO) strokes.
With the results of five separate landmark trials published in 2015 on endovascular thrombectomy (commonly called mechanical thrombectomy or endovascular clot retrieval), treatment guidelines supporting its use in LVO strokes were rapidly developed (Berkhemer et al, 2015; Gao et al, 2015). Mechanical thrombectomy is available only in specialised endovascular-capable centres, commonly termed comprehensive stroke centres (CSCs) (Gao et al, 2015).
Despite overwhelmingly high-quality evidence that demonstrates its superiority to standard medical management, however, its adoption into practice has remained slow (Pérez de la Ossa et al, 2014). This is partially a result of the complex nature of the procedure, which requires suitably qualified consultant interventional neuroradiologists.
However, an increasingly important component has been patient selection and rapid conveyance to a centre that is capable of delivering this time-dependant intervention (Pérez de la Ossa et al, 2014). Failure to detect these patients in the prehospital setting can result in significant delays, potentially jeopardising future interventions (Zhao et al, 2018).
As in the era of thrombolysis where prehospital clinical assessment tools were rapidly developed to identify strokes, recent research has focused on the development of clinical triage tools to predict mechanical thrombectomy-eligible patients in the prehospital setting (Pérez de la Ossa et al, 2016).
The purpose of this study is to assess these newer clinical triage tools, their usability, accuracy and potential implications for patients, including their role in structuring stroke systems of care.
Methodology
Search strategy
A literature search was performed on PubMed, CINAHL, Medline and Google Scholar. Key search terms included ‘stroke’, ‘prehospital’, ‘paramedics’, ‘EMS’, ‘assessment’ and ‘triage’, and were required to include the terms thrombectomy, large vessel occlusion (LVO) or clot retrieval.
Selection criteria
Six triage tools were isolated from the search results, which returned 10 triage tools for critical analysis. Although all tools offer differing perspectives on LVO triage, the decision to include tools and articles in this narrative review was based on the accuracy, development methodology and inclusion of said tool or article in any feasibility studies that were found.
Exclusion criteria consisted of any studies that were not prehospital-focused and any with poor citation metrics from journals with a poor journal impact factor. The references and linked articles were from developed countries to assess the latest response to the requirements of up-to-date stroke care practices, and all references were checked to ensure no other related research had been missed.
The review of triage tools resulted in more than 10 articles being referenced, and the analysis of systems of care included the review of over 14 articles, as well as links to the triage tools previously discussed.
Discussion
Triage tools
The ideal triage tool for suspected stroke patients would be: fast, highly reproducible, objective and with high sensitivity and high specificity for LVO stroke (Krebs et al, 2018). Additionally, a range of clinicians would have to find it easy to uses (Krebs et al, 2018).
Stroke severity has been proven to be the best predictor of LVO stroke (Krebs et al, 2018; Zhao et al, 2018). Prehospital determination of stroke severity is a new concept and, while multiple triage tools have been developed to assist in this, each tool is limited in its capacity to predict LVO strokes.
In the swift research to advance these tools, there are two unique approaches to the problem: the most popular approach is a rapid, simplistic tool (Pérez de la Ossa et al, 2014; Demeestere et al, 2017a; Noorian et al, 2018; Zhao et al, 2018); however, researchers have increasingly published comprehensive triage tools that result in significant assessment skill and prolonged on-scene times (Chen et al, 2018; Uchida et al, 2018).
Simplistic approach
NIHSS-8
The National Institutes of Health Stroke Scale (NIHSS-8) was published in 2017 and represents a cut-down version of the NIHSS designed for prehospital use (Demeestere et al, 2017a). The NIHSS is the most frequently used assessment tool for acute stroke worldwide and is commonly used by trained physicians and nurses to assess the severity of stroke symptoms.
While the full NIHSS assessment is relatively sensitive, specific and accurate in predicting stroke severity, it is not without its limitations (Pérez de la Ossa et al, 2014). These limitations are particularly relevant in the prehospital environment (Pérez de la Ossa et al, 2014). The full 15-item NIHSS stroke assessment was originally designed for research purposes; it is time-consuming and has been criticised for its complexity and redundancy, with trials that used prehospital clinicians showing significant variability and poor interrater reliability (Whelley-Wilson and Newman, 2004; Pérez de la Ossa et al, 2014).
This cut-down stroke severity scale containing eight variables was developed by retrospective analysis of NIHSS data collected on admission to the emergency department (ED) by NIHSS certified and highly experienced stroke clinicians (Demeestere et al, 2017a). Interrater reliability has been reported to be adequate with a Cohen's kappa (k) statistic of 0.69 (Demeestere et al, 2017a). Although this has been reported as ‘substantial’ agreement among raters, it must be stressed that the interrater pool of patients was n=64 and a k statistic of 0.69 could result in 35–63% of the data being unreliable (McHugh, 2012). Further attempts to simplify the scale did not improve interrater reliability (Demeestere et al, 2017b). Validation reported a sensitivity of 0.81 and a specificity of 0.75 for the detection of LVO strokes in a cohort of all possible stroke patients (Demeestere et al, 2017a). While this triage tool shows promising results, no current feasibility or prospective studies were located.
RACE
Originally published in 2013, the 5-item Rapid Arterial oCclusion Evaluation (RACE) scale is not unique in that it was developed using elements that bear a strong correlation to LVO stroke normally tested during an NIHSS exam (Pérez de la Ossa et al, 2014). Being one of the first triage scales aimed at prehospital providers and having an initially reported high sensitivity of 0.85 and a specificity of 0.65, with a cut-off point of five, this scale has been the subject of significant research. It was included in one recently published and another ongoing feasibility trial (RACECAT: NCT 02795962) (Zaidi et al, 2016; Vivanco-Hidalgo et al, 2018).
Designed retrospectively, RACE was initially prospectively validated by emergency medical technicians (EMTs) with 1 hour of additional training. Strong interrater reliability was reported, with good correlation to NIHSS assessments undertaken by neurologists once the patient was admitted to the ED (Pérez de la Ossa et al, 2014).
Limitations of this tool include the need to undertake clinical assessments above what is normally required of a prehospital clinician, although this is common and not unique to RACE.
The tool also requires cooperation with assessment to the level where language would need to be the same between examiner and patient, limiting its use in culturally and linguistically diverse populations (Pérez de la Ossa et al, 2014).
An initial data publication from Ohio, which saw a bypass protocol implemented in patients scoring a RACE of ≥5, showed that RACE is a feasible and highly effective tool with shorter door-to-recanalisation times; a trend towards improved outcomes and, importantly, no major adverse events have been reported to date (Zaidi et al, 2016). However, the generalisability of this study is limited by its unique geographical setting where there are several CSCs within a relatively small area (Zaidi et al, 2016).
LAMS
The Los Angeles Motor Scale (LAMS) scale is unique in that it is a pre-existing, rapid and well-validated stroke scale regularly used by paramedics to identify all types of strokes. In newer research, authors have attempted to validate a cut-off point that could indicate high probability of LVO stroke (Noorian et al, 2016; 2018). LAMS is a simple assessment that requires prehospital clinicians to score the severity of three deficits: facial droop; arm drift; and grip strength (Noorian et al, 2016; 2018).
Although the dataset for its latest prehospital validation was limited to 94 patients with underlying length and potential selection bias, the results were comparable with other scales (Noorian et al, 2018). With a cut-off score of >4, the results were reported as a sensitivity of 0.76 and a specificity 0.65 (Noorian et al, 2018). This prospective validation was run in parallel with study entry into FAST-MAG, which required a physician phone screen; this potentially resulted in fewer stroke mimics in the cohort (Noorian et al, 2016).
Limitations not already evident include that cortical signs, which have a strong correlation to LVO, are not assessed in this scale; however, severe motor deficits are likely to be more predictive in early stages of LVO strokes (Noorian et al, 2018). LAMS has been externally validated for LVO screening multiple times with sensitivities varying from 57–81% and specificities from 58–89% (Nazliel et al, 2008; Hastrup et al, 2016; Noorian et al, 2016; Zhao et al, 2017).
ACT-FAST
Ambulance Clinical Triage for Acute Stroke Treatment (ACT-FAST) is unique in that it presents the first algorithmic approach to clinical assessment of LVO (Zhao et al, 2018). This three-step assessment tool was designed to improve specificity by recognising only severe clinical syndromes while optimising paramedic usability and reliability (Zhao et al, 2018).
By using a sequential algorithmic approach, the authors eliminate scoring confusion that is sometimes seen in scales similar to the NIHSS. This also allows for reduced assessment time, particularly in non-LVO patients (Zhao et al, 2018). In an initial prospective validation using prehospital clinicians, the tool achieved a sensitivity of 1 with a specificity of 0.87, although this was limited by the number of patients (n=104) (Zhao et al, 2018). Its interrater reliability between doctors and paramedics is also excellent with a k statistic of 0.93 (Zhao et al, 2018).
This tool is designed with language neutrality and simplistic assessment in mind; therefore, the majority of steps can be performed on patients who present with significant deficits.
Of interest, in the prehospital clinician validation cohort, one-third of the patients did not speak English as a first language; the cohort was within the ethnically diverse population of Melbourne, Australia (Zhao et al, 2018). Important to the success of this triage tool is the inclusion of questions that can affect the eligibility of the patient to undertake mechanical thrombectomy—a first for this type of tool (Zhao et al, 2018).
ACT-FAST represents the latest in published simplistic tools for LVO identification and shows promising progression for the field; nevertheless, it requires large-scale external validation before bypass trials are considered.
Comprehensive triage tools
Representing a significantly different vision for prehospital LVO stroke identification, two research articles were published in mid-2018 that present a paradigm shift in the assessment of these patients by prehospital clinicians (Chen et al, 2018; Uchida et al, 2018). Instead of working to the commonly-held principles of rapid, easily assessed, simplistic tools, these researchers present prolonged and holistic triage tools. The tools attempt to identify specific patterns in an effort to negate the disadvantages of simple addition-based tools, which struggle to detect all interactions between variables.
Artificial neural network for LVO prediction
This clinical triage tool was developed by retrospective review of consecutive stroke patients, who underwent reperfusion therapy within 8 hours of symptom onset (Chen et al, 2018). The researchers implemented the artificial neuronal network (ANN) (a widely used system for prediction and classification of disease) and tuned it to the prediction of LVO stroke. Validation was by cross-validation rather than an external cohort and the patients were all diagnosed with acute ischaemic stroke. As such, they were not the undifferentiated cohort that is seen by prehospital clinicians (Chen et al, 2018).
The tool has 27 inputs including all 15 items of the NIHSS, age, gender, evidence of prior antiplatelet therapy and nine other risk factors (Chen et al, 2018). This approach has several advantages. For example, risk factors that are heavily associated with LVO stroke are weighed appropriately, including the presence of AF, while other items or factors are less important and weighed accordingly (Chen et al, 2018). Furthermore, this approach allows for the complex integration of concomitant factors collectively increasing the predictive power of the triage tool (Chen et al, 2018).
The researchers effectively demonstrate that, with a large number of variables, they are able to weigh each variable separately and together in order to increase the prediction accuracy (Chen et al, 2018). While sensitivity has been reported at 0.81 and specificity as 0.83, this approach is not without several limitations (Chen et al, 2018).
This novel approach is derived and validated with a single dataset of patients with acute ischaemic stroke; most evident, though, is the need for significantly more inputs than any other previously published tool. Despite the calculations being computed on the clinician's behalf, the significant time delay to undertake these assessments would be seen as deleterious in most settings (Chen et al, 2018). It could be theorised that a triage tool such as this could be used in very remote areas, where longer transport times allow for increased assessment opportunities, or in a system that has exceptional electronic care records available in the prehospital setting that could pre-populate some of the input fields. However, the increase in on-scene times to achieve marginally improved accuracy renders this approach unfeasible for most systems of care (Chen et al, 2018).
A key strength of this research piece is that additional items could be added in the future and weighed accordingly—for example, there is research into biomarkers for stroke and, with development of mobile testing, these could be easily implemented into a tool such as this (although this is not unique to this particular triage tool) (Chen et al, 2018).
Japan Urgent Stroke Triage
Based in Japan, researchers developed the Japan Urgent Stroke Triage (JUST) score—a stroke prediction tool that applies across all strokes (LVO, intracranial haemorrhage, subarachnoid haemorrhage and other ischaemic strokes) attempting to differentiate each type, which is a first for the prehospital field (Uchida et al, 2018). Similar to the ANN approach, a large number of variables are required; the input of 21 different variables makes for a significant limitation of the tool (Uchida et al, 2018). Unlike some other scales such as RACE, prehospital clinicians do not have to calculate an outcome, with a tablet computer automating this process similar to the approach of the ANN for LVO prediction (Chen et al, 2018; Uchida et al, 2018). While the tool lacks the complexity of the ANN approach (with a sensitivity of 0.69 and a specificity of 0.91 at the cut-off level of 5 or a sensitivity of 0.84 and a specificity of 0.69 at the cut-off level of 4), this tool represents good overall accuracy for LVO stroke prediction (Uchida et al, 2018).
Entirely devised and validated in Japan—a country known for low levels of multiculturalism and high life expectancy—the generalisability of this tool is unknown, despite a sample size of more than 1000 patients used for validation (Toyoda, 2013). Furthermore, interrater reliability was not tested during the validation of this tool, and external validation has yet to be undertaken.
Simplistic vs comprehensive triage tools: the paradox of increasing on-scene time
Neurons rapidly die after stroke and, with the exception of a small number of mobile stroke ambulances, effective treatments are available only in stroke capable hospitals (Zhao et al, 2017). This tight time-outcome relationship has been further strengthened with the increasing release of mechanical thrombectomy research.
Considering the strength of this data and the lack of an obvious advantage in increasing on-scene times to provide a marginally more accurate assessment, it is difficult to envisage the comprehensive approach to prehospital triage of these patients is feasible for most systems of care.
Furthermore, prehospital clinicians require significantly more training to undertake a comprehensive assessment and, while this burden is somewhat reduced with the use of technology (which is being increasingly used in the prehospital setting), the release of practising clinicians to undertake training courses remains increasingly difficult, further reducing the likelihood that a comprehensive approach is practicable.
Current recommendations
The American Heart Association (AHA) and American Stroke Association (ASA) Guidelines do not endorse bypass or directed triage (Powers et al, 2018). However, AHA/ASA's Mission: Lifeline Stroke programme, which aims to identify gaps and provide evidence-based guidelines, endorses bypass under certain conditions (Mocco et al, 2017; Leslie-Mazwi et al, 2018). It cites RACE and LAMS as suitable stroke severity tools (along with FAST-ED and CSTAT) (Leslie-Mazwi et al, 2018).
In the absence of clear evidence, the Mission: Lifeline algorithm, which was designed with the use of evidence-based guidelines, expert consensus and current practice, encourages bypass when the additional transport delay is ≤15 minutes; this may be a reasonable. approach for some systems (Mocco et al, 2017; Leslie-Mazwi et al, 2018).
Ethical dilemmas
Mechanical thrombectomy is a time-dependent intervention requiring human expertise and infrastructure that is unevenly distributed across most regions (Leira and Savitz, 2018). While the risk:benefit ratio remains uncertain, initiatives that result in the direct transfer of patients to a CSC (who as a result of prolonged transport time are now ineligible for tPA) significantly challenge the ethical principles of beneficence and justice (Gao et al, 2015; Leira and Savitz, 2018).
Furthermore, given that approximately 25% of patients are eligible for tPA and only 10% for thrombectomy, it seriously encroaches on the philosophy of utilitarianism; by bypassing a PSC, are we truly achieving the greatest good for the greatest number (Leira and Savitz, 2018)? Answers to this can only be achieved from geographically based retrospective and prospective modelling of systems of care, feasibility studies and strict clinical governance and audit processes (Garbutt and Davies, 2011; Davis et al, 2017; Ali et al, 2018).
Systems of care
It is evident that a perfect triage tool is currently unobtainable; nevertheless, with growing evidence surrounding the benefit of mechanical thrombectomy, adjustments to systems of care to deliver likely candidates for thrombectomy is urgently needed. A bypass trial has been implemented successfully in one region and with ongoing feasibility trials showing promising data, it seems feasible to advocate for bypass under specific circumstances (Zaidi et al, 2016; Vivanco-Hidalgo et al, 2018). These circumstances almost certainly need to be capacity- and geographically based, with each region assessing likely time delays to intervention, potential delays from secondary transfer and other variables, which should combine to form the ideal system of care for that location. A comprehensive system-wide assessment is required prior to any change and this is particularly relevant in the ethically challenging situation of implementing a feasibility trial (Table 1).
| Variables for an appropriate thrombectomy systems-of-care assessment |
|---|
| Primary stroke centre (PSC) locations |
| Comprehensive stroke centre (CSC) locations |
| CSC capacity |
| Potential for PSC–endovascularly equipped (PSC-EE) hospitals (Trip-and-treat capable) |
| Secondary transfer delays |
| Primary transfer time-saving |
| Prehospital clinician education levels and existing assessment skills |
| Ambulance service capacity |
| Process performance (including door-to-recanalisation times) |
With varying degrees of sensitivity and specificity, it could be reasonable that a system with long transport times would bypass a PSC only with a highly specific tool, even at the detriment of sensitivity, while a system that is predominantly urban can afford to bypass a PSC with a tool that is highly sensitive, even with loss of specificity (Pérez de la Ossa et al, 2016). False positives will exist in all scales, although the majority are likely to be patients who may benefit from admission to CSCs regardless, such as those with subarachnoid haemorrhages (Pérez de la Ossa et al, 2014; 2016; Zhao et al, 2018) (Table 2).
| Triage tool | Reported sensitivity* | Reported specificity* |
|---|---|---|
| Simplistic | ||
| National Institutes of Health Stroke Scale-8 | 81% | 75% |
| Rapid Arterial oCclusion Evaluation (RACE) Scale | 85% | 65% |
| Los Angeles Motor Scale (LAMS) Scale | 76% | 65% |
| Ambulance Clinical Triage for Acute Stroke Treatment (ACT-FAST) | 100% | 87% |
| Comprehensive | ||
| Artificial neural network for LVO prediction | 81% | 83% |
| Japan Urgent Stroke Triage (JUST) Score (cut-off at 4) | 84% | 69% |
| Japan Urgent Stroke Triage (JUST) Score (cut-off at 5) | 69% | 91% |
Dependent on an accurate assessment of the system, the ideal model is likely to be a combination of the commonly touted approaches of drip-and-ship, mother-ship and the newer emerging system of trip-and-treat (Figure 1).
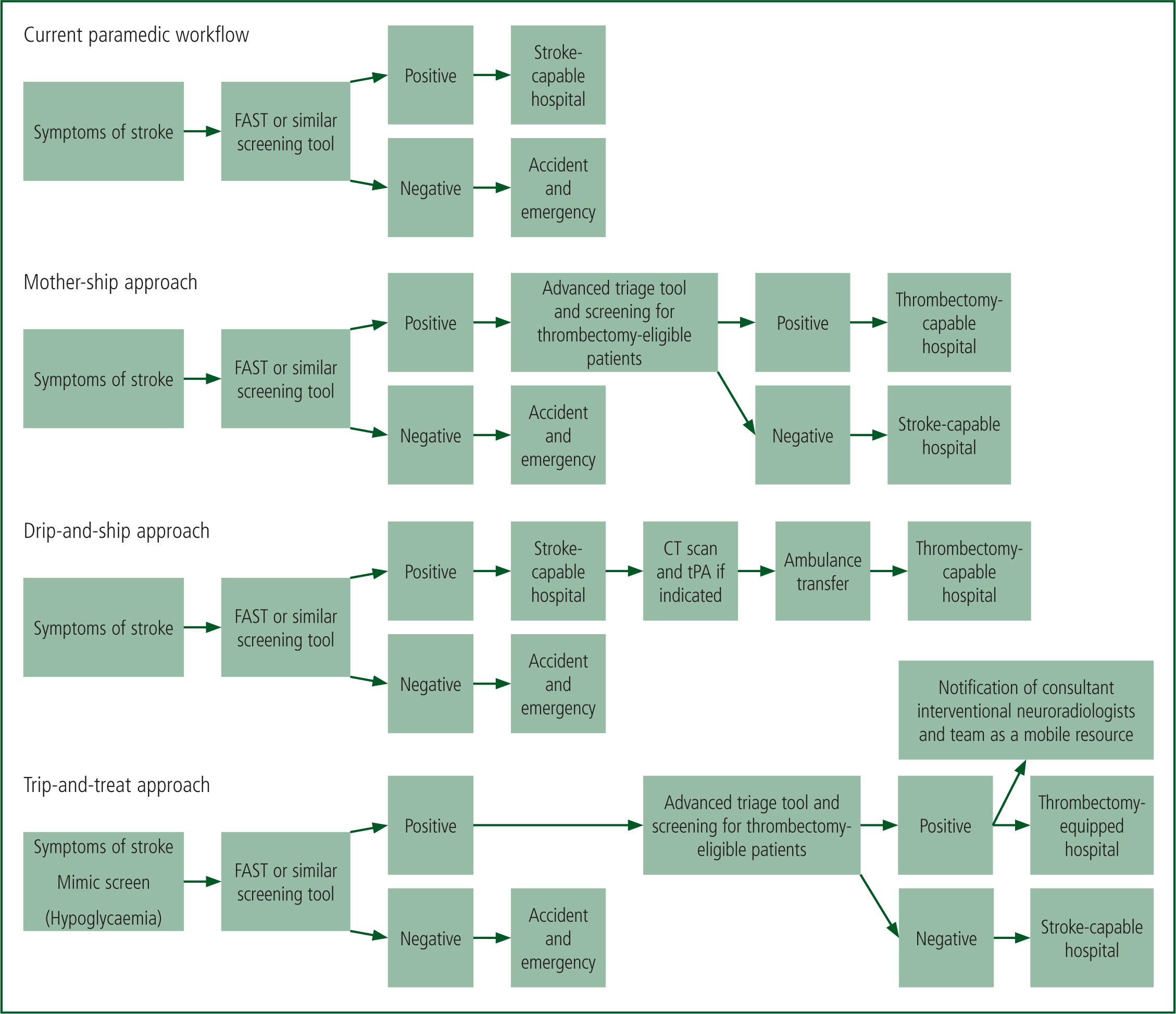
The two most commonly implemented systems of care are the drip-and-ship and the mother-ship approach (Ali et al, 2018). The drip-and-ship model involves the patient being rapidly conveyed to a PSC to minimise time to tPA before being transferred to a CSC for endovascular procedures where indicated (Leira and Savitz, 2018). In the mother-ship approach, the patient is conveyed directly to the CSC for tPA and/or endovascular procedures (Leira and Savitz, 2018). Each approach's advantage is the disadvantage of the other; with drip-and-ship, fewer patients will reach the CSC outside the tPA eligibility window, whereas in the mother-ship approach, longer transport times could theoretically exclude patients from tPA treatment, as well as potentially cause capacity issues at the CSC.
The emerging trip-and-treat model of care involves interventional stroke teams becoming a mobile resource that is deployed to primary stroke centres equipped to allow interventional procedures; this could suit some systems, particularly in rural areas (Wei et al, 2017; Hui et al, 2018). Trip-and-treat’ could result in the system allowing 24/7 interventional capacity where this was previously limited, improving geographical coverage as well as significantly improving recanalisation times. In a recent proof-of-concept study, door-to-recanalisation was 79 minutes faster for the trip-and-treat cohort of patients compared with the drip-and-ship approach (Wei et al, 2017).
Although trip-and-treat appeals as a potential fix-all approach, in reality, most hospitals lack adequate resources for endovascular procedures and trip-and-treat would be difficult to implement in non-integrated healthcare systems (Wei et al, 2017).
Limitations
This narrative review focused on prehospital triage tools and their implications in the systems of stroke care. Articles were excluded that did not mention prehospital involvement and, as such, the analysis regarding changes to systems of care could lack balance. Only English language articles or those translated to English were examined and therefore the possibility of language bias exists. Some triage tools were excluded based on the lack of up-to-date research. While this is generally positive, as this area of research is in rapid development, some current triage tools may have been overlooked.
Conclusion
Patients with LVO strokes are best managed at CSCs; prehospital triage tools may help in directing transport decisions for these patients. As we obtain stronger evidence of the effectiveness of endovascular approaches to ischaemic stroke management, the importance of prehospital recognition increases. The process of bypassing PSCs using existing triage tools appears feasible and has been proven in one study; theoretical delays to the treatment of all other patients would need to be ethically assessed in each system of care and this is particularly geographically specific.
The advantages of direct transport are clear, with a reduction in revascularisation times, which has been strongly associated with improved outcome in these patients. In addition, the emergency medical system sees a reduction in secondary transfers. Together, these advantages make the mother-ship approach appealing, both clinically and logistically.
Although comprehensive triage tools are assisting the prehospital community to understand the complexity of stroke triage, it is unlikely that prolonged complex triage tools are the answer to rapidly delivering appropriate patients to appropriate stroke centres.
As the development of triage tools advances, the concurrent development of triage paradigms and protocols needs to be prioritised. Changes to systems of care should be locally driven, with analysis of variables extensive; generalisation of other systems of working will be difficult, with geographical profiles being a major compounding factor. While over- and under-diagnosis is inevitable, strong locally-based evidence and robust ethical management will limit liability.

