Participation in endurance events is popular, yet not without risk; a number of individuals will become ill with a fatality rate 0.4–3.3 per 100 000 entrants (Schwellnus et al, 2019). Endurance events such as running, cycling and triathlons present unique challenges to prehospital providers who may treat patients at these events when working either at the event or in their normal prehospital role in the area nearby.
In addition to illnesses such as cardiac arrest, arrhythmia and seizures that can be encountered, there are a number of conditions unique to endurance sports, which include exercise-associated collapse (EAC), exertional heatstroke and exercise-associated hyponatraemia (EAH).
Assessment and management of these patients can be difficult because of similarities in their initial clinical presentation and condition complexities. In this article, common causes of collapse unique to endurance sports medicine are discussed and an assessment framework is proposed for practitioners who may encounter these conditions. This proposed framework takes account of pathologies unique to sports medicine as well as other causes of collapse.
Medical conditions
The are several medical conditions unique to exercise. While this article discusses collapse from the perspective of the athlete, exercise-related conditions can occur in others who are exercising as part of work or recreation.
Table 1 shows medical conditions that are unique to exercise and conditions that may occur during exercise; many of these can be precipitated by the physiological changes and stress seen during exercise. The causes that are unique to exercise will be discussed further.
| Unique to exercise | May occur during exercise |
|---|---|
| Exercise-associated collapse | Sudden cardiac death |
| Exertional heatstroke | Myocardial infarction |
| Exercise-associated hyponatraemia | Subarachnoid haemorrhage |
| Hypoglycaemia | |
| Epilepsy |
Exercise-associated collapse
EAC is the most common condition practitioners are likely to encounter when treating patients who have participated in an endurance event. Estimates of incidence vary; Roberts (2000) reported that, over a 12-year period at the Twin Cities Marathon, 59% of patients they interacted with had EAC.
The majority of cases of EAC occur after the athlete has finished their activity so cases cluster at the finish line.
The principal mechanism responsible for EAC is thought to relate to the combined effect of peripheral vasodilation and the redistribution of blood to the lower limbs. On stopping exercise, the muscle pump that returns blood from the extremities significantly reduces effort, with an associated fall in venous return and consequently cardiac output (Noakes, 2007).
An additional key contributing factor is the effect of an impaired baroreflex response. During exercise, participants are typically vasodilated; on stopping, there is a need for baroreceptors to maintain adequate systemic vascular resistance through vasoconstriction. If this response fails because the baroreflex is impaired, collapse after exercise is more likely (Asplund et al, 2011).
The majority of patients with EAC will recover quickly with simple measures including lying supine or, ideally, in the Trendelenburg position and then drinking fluid. A small study comparing outcome differences between patients with the above measures and those treated with intravenous fluids showed there was no difference in rates of recovery (Anley et al, 2011). This not only has clinical implications for individual providers but also correlates with the current understanding of the underlying pathology. Furthermore, the fact that the majority of patients recover without intravenous fluids should be borne in mind by all who work at endurance events. Injudicious use of intravenous fluids may precipitate symptomatic EAH, so these should not be routinely administered to the collapsed endurance athlete.
While EAC is the most common cause of collapse in the endurance athlete, practitioners should remain vigilant to some of the less common but life-threatening alternative differentials such as heatstroke and EAH.
Asplund et al (2011) recommend a framework for assessment of the patient likely to have EAC; this includes lying the patient down, encouraging oral fluid use and then reviewing in 15–20 minutes. Patients who show no improvement after this time or still present with abnormal mental status, tachycardia and or hypotension should be cause for concern and prompt more aggressive assessment and investigation. Those who show improvement, are able to ambulate and have a normal mental status can be considered for discharge.
Exertional heatstroke
Exertional heatstroke (EHS) is a life-threatening condition associated with endurance activities such as those undertaken by endurance athletes and members of the military (Périard et al, 2022). The key features of EHS are a core temperature of >40°C and neurological impairment. Untreated, its mortality may be up to 80% (Walter and Steel, 2018).
Establishing the exact incidence of EHS is difficult owing to the limitations of reporting. However, incidents are high enough that it should be a key differential for prehospital providers. Data from a 5-year period at the Boston Marathon demonstrated an incidence of 3.7 cases per 10 000 starters. At the UK Great North Run (a half marathon), there were 55 cases of patients with a core temperature >41°C (Hawes et al, 2010). In the United States, EHS has been observed in high school athletes with at a rate of 1.2 per 100 000 (Kerr et al, 2013).
Patients with heatstroke lose their ability to thermoregulate. As thermoregulation is lost, multiple pathological processes occur. One key process is increased gastrointestinal permeability (sometimes known as the leaky gut hypothesis), which results in toxins from the gastrointestinal tract moving into the bloodstream resulting in a worsening of the global inflammatory response and potentially contributing to ongoing hyperpyrexia and multiorgan dysfunction. Disseminated intravascular coagulation and associated coagulopathy can result from this dysfunction (Garcia et al, 2022).
A number of patients with EHS will have concurrent exertional rhabdomyolysis. This can occur independently of EHS and may be identified with an isolated rise in serum creatinine kinase or with more profound acute kidney injury. In both EHS and exertional rhabdomyolysis, a hypermetabolic state is the underlying pathology (Kruijt et al, 2023).
Like many life-threatening conditions associated with the collapsed athlete, EHS is associated with an altered level of consciousness. Consequently, on clinical assessment, it can be hard to differentiate EHS from other conditions such as hypoglycaemia and EAH. Examination findings are of limited use in making a diagnosis of EHS. In classical non-exertional heatstroke, the skin is dry but, in EHS, the patient may be sweating. Additionally, skin colour may vary from flushed to pale depending on whether the patient is vasodilated or in a state of cardiovascular collapse (Epstein and Yanovich, 2019).
The key investigation for the diagnosis of heatstroke is measurement of core temperature. Measures of temperature that are non-core, such as oral or tympanic, are unreliable in the athlete who may have heatstroke (Morrissey et al, 2021) and should not be used to measure temperature in the collapsed athlete. The simplest and most readily available method to measure a core temperature in the prehospital environment is recording a rectal temperature. It is vital that a core temperature is recorded rapidly in any collapsed athlete to identify patients with EHS and facilitate rapid treatment. Providers should not be falsely reassured by a normal peripheral temperature.
If patients are found to be hyperthermic, aggressive cooling should be initiated. Practitioners working at events should consider in advance how they will rapidly actively cool this cohort of patients at any location on the event course. The most effective method is immersion in cold water or relying on convective heat loss by using cold water sprays and fanning (Gaudio and Grissom, 2016). Ice water immersion, while very effective, has practical limitations such as not being universally available. Additionally, there are concerns that it is difficult to monitor patients fully and it may prove challenging to provide additional support such as airway management. Spraying with cold water and fanning are not as efficient as immersion but do allow full monitoring and can be performed anywhere on the event course and in an ambulance during transfer.
The UK's Faculty of Sport and Exercise Medicine recommends that cooling is continued until the core temperature is between 38.5°C and 39°C. Once this target is reached and confusion has resolved, active cooling should be stopped to avoid the risk of overshooting and inducing hypothermia (Walter et al, 2018). On occasion, hypothermia may be a cause of collapse, particularly in slower participants and in inclement weather.
Exercise-associated hyponatraemia
The other exercise-specific differential prehospital health professionals may encounter when attending the collapsed athlete is EAH. EAH is defined as a sodium level of <135 mmol/l during exercise or up to 24 hours after exercise (Scheer and Hoffman, 2018). It is a complex process with several contributing factors. Excessive sodium loss through sweat can cause hyponatraemia (Seal and Kavouras, 2021). However, the two main factors that cause hyponatraemia in athletes are excessive water intake and secretion of antidiuretic hormone in response to exercise (Hew-Butler et al, 2015; Seal and Kavouras, 2021).
Overconsumption of hypotonic fluid is a principal driver of EAH, particularly when athletes are given inappropriate advice to consume more fluid than required. Consequently, to prevent EAH, participants in endurance events should be advised only to drink to thirst to avoid overconsumption of hypotonic fluid and prevent consequent EAH (Hew-Butler et al, 2017).
Antidiuretic hormone, also known as arginine vasopressin, is one of the key hormones in regulating the body's fluid balance. It is released when serum osmolality increases beyond a set point, promoting water reabsorption in the kidney (Seal and Kavourus, 2022). A number of stimuli can cause excess secretion of antidiuretic hormone in athletes, including physical exercise, pain, distress, nausea and exposure to heat (Hew-Butler et al, 2017).
Medication that participants may be taking can also be a risk factor for hyponatraemia. An example is non-steroidal anti-inflammatory drugs through the potentiation of antidiuretic hormone (Whatmough et al, 2018)
Asymptomatic EAH is relatively common; in a study of 88 participants at the London Marathon, there were 11 (12.5%) cases of asymptomatic hyponatraemia (Kipps et al, 2011). Incidents were similar at the Boston Marathon, where 13% of a cohort of 488 patients were hyponatraemic (Almond et al, 2005). Higher numbers have been reported in the Spartathlon Ultramarathon, with 65% of a cohort sampled being hyponatraemic (Seal et al, 2019). This is probably a reflection of the duration, terrain and temperature changes seen in the race (Seal et al, 2019). EAH has been reported in shorter events, including half marathons (Glace and Murphy, 2008).
The incidence of symptomatic hyponatraemia is significantly less in comparison, with fewer than 1% of endurance athletes experiencing symptomatic hyponatraemia (Noakes et al, 2005).
Mild symptoms of EAH include dizziness, light-headedness, fatigue and nausea. More severe symptoms include headaches, confusion, poor coordination and seizures; these more severe symptoms may indicate cerebral oedema and should be regarded as life threatening (O'Connor, 2006).
The collapsed athlete may exhibit limited signs to indicate they are hyponatraemic, particular if obtunded.
In addition to the neurological sequelae of hyponatraemia, patients with severe EAH can develop signs of respiratory distress, with frothy sputum production secondary to non-cardiogenic pulmonary oedema (Hew-Butler et al, 2015).
The increased awareness of EAH and difficulties in diagnosis have led to point-of-care testing being provided at many large endurance events so people with hyponatraemia can be rapidly identified and treated.
Isotonic fluids such as 0.9% sodium chloride can precipitate asymptomatic hyponatraemia progressing to symptomatic hyponatraemia (Hew-Butler, et al 2015). Consequently, before isotonic intravenous fluids are administered to a collapsed athlete, every effort should be made to measure serum sodium. If serum sodium cannot be recorded, senior clinical advice should be sought before the administration of any intravenous fluid because of this significant risk.
If a patient has severe symptoms of hyponatraemia such as confusion or seizures, treatment with hypertonic sodium chloride should be initiated. Furthermore, if the index of clinical suspicion is high for EAH and point-of-care testing is unavailable, hypertonic saline boluses should be administered empirically.
The recommended dose for hypertonic saline is 100 ml of 3% sodium chloride, repeated twice if there is no clinical improvement (Hew-Butler et al, 2015). Alongside the specific treatment of hypertonic saline, supportive care should be provided.
To facilitate neurological protection, some patients will require prehospital emergency anaesthesia, which needs to be provided by a suitable critical care team.
An approach to assessment
Differentiating the cause of collapse in the context of the endurance athlete is challenging as clinical presentations are often similar, with the majority of collapsed runners appearing pale and diaphoretic. Additionally, collapsed participants are often tachypnoeic and tachycardic because of either the recent intense exercise or the acute pathology.
One of the most important clinical features of which providers must be mindful is confusion: while it does not indicate a particular pathology, it should be seen as a red flag for serious pathology and prompt urgent assessment of the patient.
The algorithm in Figure 1 outlines an approach to rapidly differentiating between the various causes of collapse in an endurance athlete.
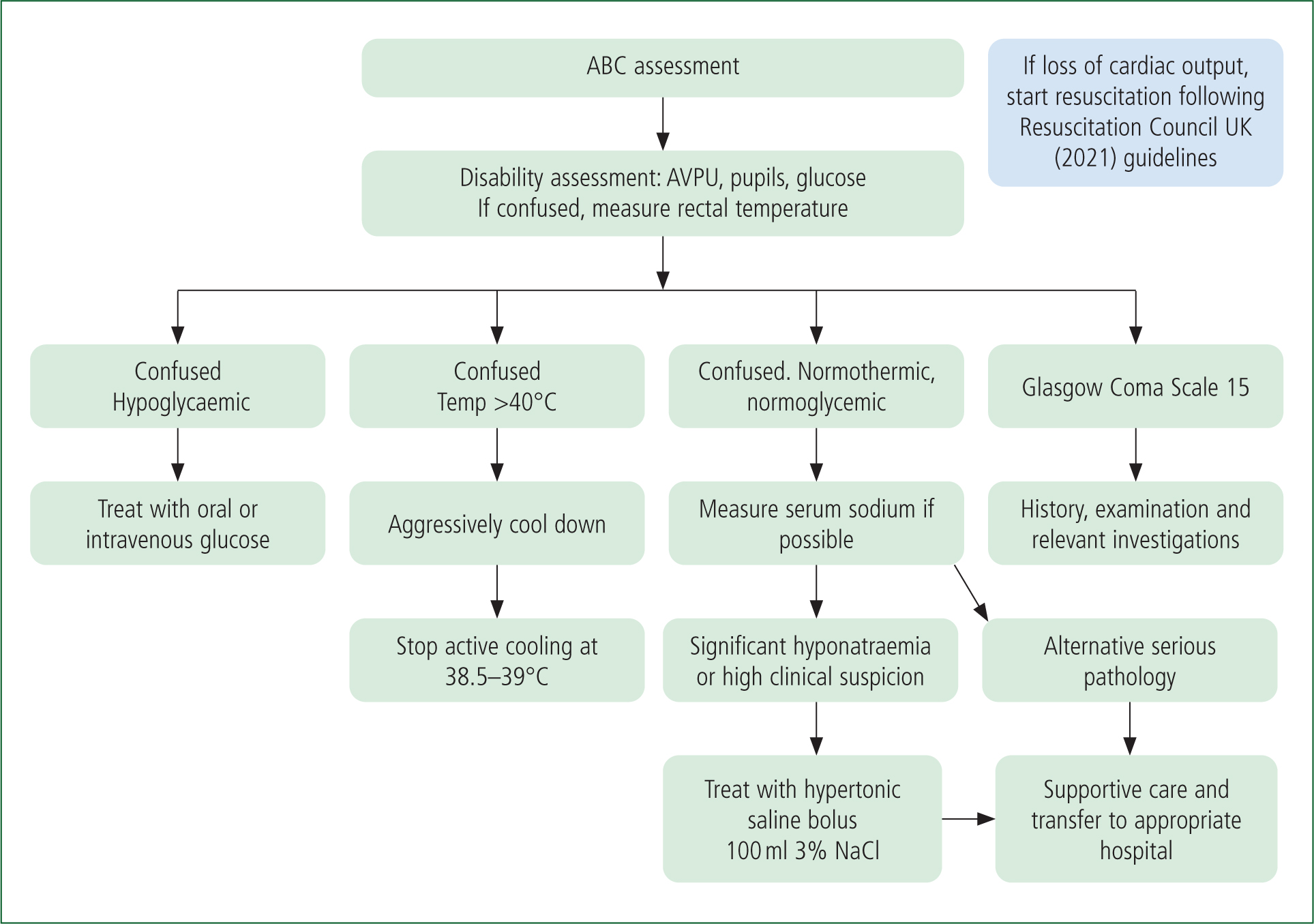
Initially, providers should carry out a primary survey and measure baseline vital signs. A small number of patients will be in cardiac arrest and resuscitation should be instigated immediately. To facilitate this, event planners need to ensure the provision of staff and equipment including defibrillators throughout the course of an event.
Given that many patients who collapse during or immediately after exercise appear critically ill and pulse oximetry may not be reliable, it is reasonable if concerned to administer high-concentration oxygen during the initial phase of their management.
As stated, the majority of people who have collapsed at endurance events will be experiencing EAC. It is therefore preferable to assess patients lying down and, if possible, with their legs elevated. While patients with EAC may well be initially hypotensive, this tends to rapidly correct with lying in this position. In the absence of concerning factors and following a short period of rest and oral fluids, these patients can generally be safely discharged. Failure to rapidly improve should prompt practitioners to undertake a more detailed examination (Asplund et al, 2011) and to complete investigations such as a 12-lead electrocardiogram and serum sodium measurement as well as to consider other causes such as acute coronary syndrome.
It is important to rapidly assess a patient's level of response; confusion in a runner who has collapsed should be viewed as a red flag and mandates measurement of a rectal temperature to diagnose or exclude EHS. Additionally, a capillary blood glucose level should be measured. Altered mental state and a core temperature >40°C is diagnostic of heatstroke (Walter and Steel, 2018). Having made the diagnosis of heatstroke, it is imperative to rapidly cool the patient in the prehospital setting to prevent death and multiorgan dysfunction (Filep et al, 2020).
If an athlete has collapsed and is confused but has a normal or mildly elevated core temperature, other causes should be considered. EAH is the potentially life-threatening condition specific to exercise that practitioners should be mindful of in a case of collapse and confusion in the endurance athlete. Confusion and a normal temperature should prompt practitioners to record a serum sodium level and manage EAH accordingly.
At many events, patient details and past medical history are given on the back on the participant's number. This number should also be documented in the patient record and accompany the patient if transferred to hospital to ensure they can be identified and their family contacted if required. However, it should be noted that some participants may swap their race number with friends or relatives, even though this is against race rules (Tunstall Pedoe, 2007).
While the assessment algorithm shown in Figure 1 focuses on the common causes of collapse in an endurance athlete, health professionals should be mindful that other differentials such as subarachnoid haemorrhage, acute coronary syndrome, hypoglycaemia and epilepsy can all precipitate collapse. Having completed a primary survey, the major decision point with respect to assessment comes at identifying confusion. As stated above, the two causes of collapse unique to exercise that are associated with confusion are EHS and EAH. If these have been excluded and the patient is normoglycaemic but remains confused, they should be assumed to have other severe underlying pathology such as a subarachnoid haemorrhage or shock causing poor cerebral perfusion. They should receive further assessment and supportive care and be promptly transferred to hospital.
Conclusion
Prehospital practitioners who work at endurance events or see patients taking part in these events need to be aware of several differential diagnoses unique to endurance activities and have a systematic approach to assessment and management. One proposed method for assessment has been set out in this article.
The majority of participants who collapse at endurance events will have EAC and, with simple supportive care, most will recover quickly and can be safely discharged. However, it is important that prehospital practitioners are able to identify a number of other causes. Confusion should be considered a red flag and prompt assessment for EHS, EAH and other serious pathologies. If EHS is identified, the patient should be rapidly cooled. Patients with symptomatic EAH should receive hypertonic sodium chloride.
With the growing popularity of endurance events, it is likely that prehospital practitioners will see more of these patients and the conditions discussed. Highlighting these conditions and potential differentials that need to be considered will raise awareness and may encourage further research on incidence, risk factors and optimum management for each of them.

