LEARNING OUTCOMES
After completing this module, the paramedic will be able to:
If you would like to send feedback, please email jpp@markallengroup.com
It's a cold and wet Saturday afternoon when you are dispatched to a 9-year-old male with a suspected lower limb injury. The incident location is a local football field. On your arrival, you are greeted by the coach and a few other parents who inform you that he has twisted his ankle badly. Owing to the weather conditions, you take the stretcher, scoop, Entonox®, vacuum splint and a few blankets onto the pitch in anticipation of moving to the ambulance. You find the patient in the supine position with his hands covering his face, screaming. The history of the complaint involves the patient entering a tackle with another player; he heard a ‘crack’ and felt a sudden surge of pain to his right ankle. Unable to weight-bear, he remained on the floor. You undertake a quick primary survey and then expose and examine the ankle which is swollen and painful to touch. The pedal pulse is present; there are no breaks to the skin; and distal movement and sensation are intact. You suspect a fractured distal tibia/fibular on account of the significant swelling. A pain score is obtained (10/10) using the Numeric Pain Rating Scale (NPRS). You make the decision to administer Entonox, apply a vacuum splint, scoop onto the stretcher and move onto the back of the ambulance to escape the adverse weather conditions.
You obtain clinical observations which can be seen in Table 1. Having moved the patient, you re-examine the ankle to ensure there is no limb threat or open fracture. You examine for distal circulation, movement, sensation and for broken skin or protruding bone. You then perform a full top-to-toe examination to rule out other injuries. Having initially managed the pain with Entonox and still scoring the pain 7/10, with parental permission you gain intravenous (IV) access, check your Joint Royal Colleges Ambulance Liaison Committee (JRCALC) guidelines (Brown et al, 2016), and administer 3 mg morphine sulphate IV. After a further 10 minutes, the patient's pain has reduced to 3/10 and he appears much more comfortable. You convey the patient to the local emergency department accompanied by his mother. You take a final pain score on arrival at hospital which has remained unchanged at 3/10.
| Observation | Result |
|---|---|
| Respiratory rate | 24 breaths per minute |
| Heart rate | 130 beats per minute |
| Blood pressure | 110/78 mmHg |
| Temperature | 36.2 °C (tympanic) |
| Oxygen saturations | 100% |
| Glasgow coma scale | 15/15 |
| Electrocardiogram | Sinus tachycardia |
| Blood glucose | 5.2 mmol/litre |
| Pain score (second) | 7/10 NPRS |
What is pain and why do we need to treat it?
Pain is ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage’ (International Association for the Study of Pain (IASP), 1994). A number of theories seek to explain the complex phenomenon of pain. Melzack and Wall (1965) proposed gate control theory, which is arguably the most accepted theory, combining earlier concepts of specificity and pattern theory.
This theory explains that pain is controlled by a ‘gate’ and that certain factors will open and close the gate. Gate control theory accepts the disparity between pain stimuli and pain perception, and that psychological factors, e.g. previous experience of pain, level of concentration, and emotion, can influence the ‘gate’, and subsequently affect the amount of pain perceived.
According to international human rights law (Lohman et al, 2010) and the World Health Organization (WHO) (2015), all countries must provide pain treatment medication as a core obligation under the right to health care.
The management of pain is complex—especially in children (aged below 18 years)—as age, developmental level, cognitive and communication skills, and associated beliefs must be considered (Srouji et al, 2010). Pain can have psychological, physical and social consequences which impact quality of life (Lohman et al, 2010). Without effective pain treatment, children may suffer longterm changes in stress hormone responses and pain perception (Finley et al, 2005), and are at risk of developing post-traumatic stress disorder (PTSD) (Saxe et al, 2001; Sheridan et al, 2014).
Common causes of pain in children
The pre-hospital evidence base surrounding pain management in children is limited within the UK. The current article has therefore used an array of international evidence and resources that best reflect current practice.
A recent United States (US) study involving 14 emergency medical services found that from 55 642 calls to patients under 19 years of age, traumatic injury was documented as the presenting complaint in 26% of cases, making it the most frequent presenting complaint (Lerner et al, 2014). It also found that ‘pain non-chest/non-abdomen’, ‘abdominal pain/problems’ and ‘chest pain/discomfort’ were documented in 10.5%, 4.1% and 1.5% of cases, respectively.
Although trauma is the most common cause of pain in children in the pre-hospital setting, causes of abdominal pain are also frequent and important to remember. The following are a number of common abdominal pain causes in children (adapted from Victorian State Government, 2013):
In addition to identifying the cause of pain, pre-hospital clinicians have a duty to recognise signs of potential abuse in children. This includes emotional, sexual and physical abuse, and neglect (Brown et al, 2016). Suspicion of physical abuse should be considered in the presence of:
These examples are by no means exhaustive and where abuse or neglect is suspected, a safeguarding referral should be made according to local guidelines. The receiving department should be made aware of the suspicion and referral.
Assessing children suffering pain
There are a number of mnemonics that can assist in the assessment of pain. Some examples are:
An in-depth assessment of the pain can help indicate its aetiology and assist in the development of a clinical impression. It is important to keep an open mind when assessing pain and explore all possible avenues, as it's easy to get ‘tunnel vision’ and focus on the most likely cause.
Pain is a complex and difficult symptom to assess because each patient will experience pain differently depending upon their age, gender, prior pain experiences and cultural/social norms (Lord, 2015). It's also important not to let our own perception of pain influence our assessment, as paramedics are likely to underestimate pain (Solomon, 2001).
Measuring pain in children
Measuring a child's pain is paramount. Without measurement, you cannot hope to manage the pain adequately because you have no baseline against which to measure whether the management has been effective or not. With the given scenario, it would have been tempting to quickly administer the Entonox and transfer to the ambulance before taking a pain score. However, without the pre-analgesic pain score, the effectiveness of Entonox would be unknown. Therefore, the clinician would not be able to make an informed decision regarding the next step of pain management.
Use of age-appropriate tools
An age-appropriate pain management tool should be used. This promotes compliance of self-reporting and prevents the clinician ‘estimating’ the child's pain. Despite there being no pain assessment tools for children validated in the pre-hospital setting (Brown et al, 2016), it's acceptable to use tools that have been validated in-hospital.
For this scenario, the 11-point NPRS was used because of the patient's age and cognitive ability. This tool should be understood and easily interpreted by children aged 8 years and older (von Baeyer et al, 2009). Older children may also use the Adjective Response Scale (ARS) where children give a verbal response of ‘none’, ‘slight’, ‘moderate’, ‘severe’, or ‘agonising’ (Lord, 2015). However, this tool is limited by cultural and language barriers, and is less sensitive to small changes in pain. The Visual Analogue Scale (VAS) is well-validated in children aged 6 years and over (Ho et al, 1996). The 10 cm line is a common version with the words ‘no pain’ and ‘worst pain’ at either end of the scale. The patient is then asked to point to where their pain matches the line.
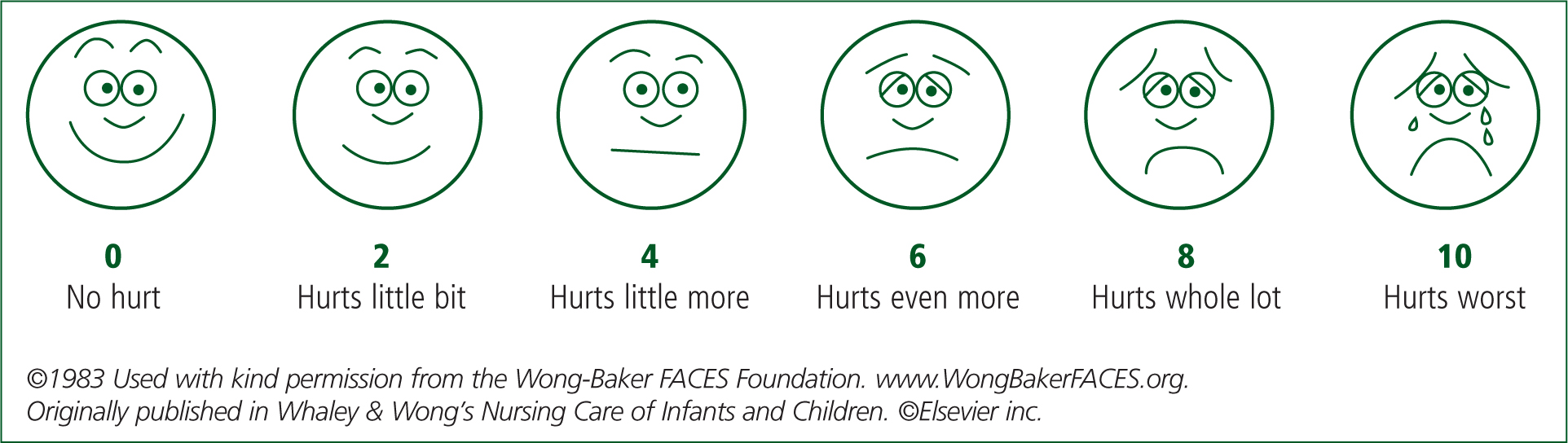
For children aged 3 years and older, the Wong-Baker FACES Foundation (2017) scale is a validated tool that can be used (Figure 1). This tool has been developed and revised over a number of years and now coincides with the 11-point NPRS with scores of 0, 2, 4, 6, 8 and 10 as opposed to the original 0–5.
The Oucher!TM scale (Beyer et al, 2009) combines the VAS and the Wong-Baker concept to create a multifunctional tool for children aged 3–12 years. This tool is unique as the pictures are of real children and a variety of ethnicities are available to choose from.
Finally, for pre-verbal or older children with reduced cognitive ability, the FLACC [Face, Legs, Activity, Crying and Consolability] (Merkel et al, 1997) scale is a useful objective tool (Table 2). This results in a pain score of between 0 and 10.
| Score | |||
|---|---|---|---|
| Behaviour | 0 | 1 | 2 |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent to constant quivering chin, clenched jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting, back and forth, tense | Arched, rigid or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers; occasional complaint | Crying steadily, screams, sobs, frequent complaints |
| Consolability | Content, relaxed | Reassured by touching, hugging or being talked to, distractible | Difficult to console or comfort |
Another useful pre-verbal pain scale is EVENDOL (Fournier-Charriere et al, 2012), which has been validated within the emergency department for use with children aged 7 years and under.
When measuring pain, it's important to know when a ‘clinically meaningful’ reduction in pain is achieved. A pain score reduction of 2 or more out of 11 is often deemed effective or ‘clinically meaningful’ (Farrar et al, 2001; Bulloch and Tenenbein, 2002; Farrar et al, 2003; Myrvik et al, 2013; Whitley and Bath-Hextall, 2017). However, given this clinical scenario, even though a pain score reduction of 3 was achieved with Entonox, which is clinically meaningful, this was not a reason to stop and not progress up the ‘analgesic ladder’ (WHO, 1986), as the patient was still suffering severe pain.
Managing pain adequately
Pharmacological
The analgesic ladder was first proposed by the WHO (1986) for adult patients suffering cancer. Since its inception, other health-care fields have adopted this ladder, including ambulance services. The ladder proposed a systematic approach to managing pain, starting with non-opioid medication and progressing to weak and finally strong opioid medication if the pain persists or increases. Since then it has been proposed that for children suffering persistent pain from medical illness a ‘two-step’ approach is to be taken (WHO, 2012). This involves:
This negates the need for a mild opiate, such as codeine, and mitigates the delay in administering strong opiates for moderate to severe pain when using the original ‘ladder’ approach. This approach is arguably more relevant to pre-hospital pain management, however, this recommendation is based on ‘very low quality’ evidence and is out of context—therefore further research would be ideal.
A number of medicines are available to paramedics which can be used for the treatment of pain. These include paracetamol tablets, paracetamol suspension, ibuprofen tablets, ibuprofen suspension, Entonox, oral morphine and IV morphine sulphate. Other medicines may be available through local patient group directions.
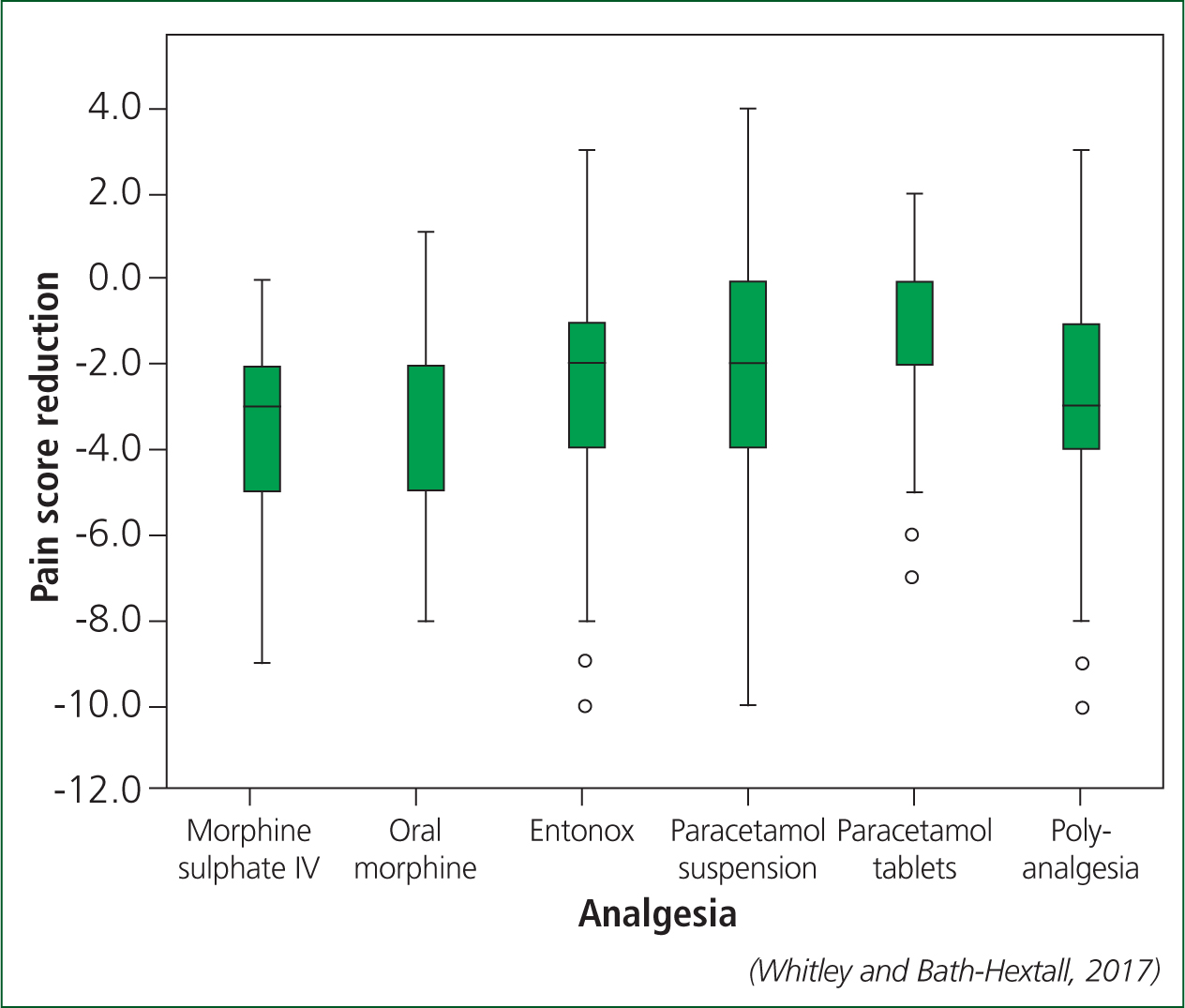
A recent study determined the clinical effectiveness of these medicines when treating children with injuries and found that they all produced a clinically meaningful reduction in pain with the exception of paracetamol tablets (ibuprofen was not included) (Whitley and Bath-Hextall, 2017) (Figure 2).
However, there are advantages and disadvantages to a number of these medicines. Paracetamol and oral morphine can be administered with ease; yet both are slow-acting drugs, especially oral morphine owing to gastric wastage and reduced absorption times (Brown et al, 2016). Oral morphine has a bioavailability of 23.9% (Hoskin et al, 1989; Halbsguth et al, 2008) and maximum plasma concentrations are seen at 45 minutes post administration (Hoskin et al, 1989). Although a potent analgesic, oral morphine takes a significant time to reach its peak effect. Considering the low bioavailability, administering 10 mg of oral morphine is roughly equivalent to 2–3 mg of IV morphine.
IV morphine sulphate has a bioavailability of 100%, and takes effect within a minimum of 2–3 minutes, with the maximum effect taking place between 10 and 20 minutes (Brown et al, 2016). However, the process of peripheral intravenous cannulation is painful and notoriously difficult in paediatrics, especially younger children (Reigart et al, 2012). Entonox is an effective analgesic, quick and easy to administer with its maximum effect taking place between 2 and 3 minutes, and the effects wear off within 30 minutes (BOC Healthcare, 2015). However, some may find Entonox inadequate as one study showed that inhaled Entonox was effective in the majority of patients (80.5%), leaving the remainder in pain (Heinrich et al, 2015). Furthermore, inhaled analgesics are difficult to administer to distressed and uncooperative children (Murphy et al, 2014).
It is important to remember that while children are not small adults, often the effects of drugs on children are similar to that of adults (Stephenson, 2005). Nonetheless, there are a number of pharmacokinetic differences that separate children and adults, meaning the same dose per kg cannot be transcribed from adults to children. A number of these differences are (Stephenson, 2005):
Therefore, it is of the utmost importance that local and national guidelines are followed when calculating analgesic dosages in children.
Non-pharmacological
This form of intervention is extremely potent in children and should never be overlooked or underestimated. Types of non-pharmacological intervention include:
The key here is accurate documentation. The use of distraction may seem minor or even just natural, and may therefore be overlooked as an important intervention to document; however, documentation of pain scores along with the use of non-pharmacological interventions helps to create a more informative and complete clinical record.
Accurate documentation
As the saying goes: ‘if you don't document it you didn't do it’. The most important aspect of clinical practice is to treat the patient to the best of one's ability; however, it is of the utmost significance that the pain management process is documented accurately. A recent evaluation of pain management in children showed that from 2596 clinical records of injured children in pain, 1663 (64%) could not be used because the vast majority had no pre- or post-analgesic pain score documented (Whitley and Bath-Hextall, 2017). Without a pre and post-intervention pain score, the effectiveness of the intervention is unknown, and is therefore of little use to the clinicians being handed over to, and to any audit or future research that the clinical record may be involved in.
Conclusion
Despite pain assessment, measurement and management being challenging in the pre-hospital child, there are ways to manage the process effectively. Performing an in-depth assessment of pain can help to identify the correct aetiology; using an age-appropriate pain scale will promote accurate pain-reporting; and managing the pain via a tailored mix of pharmacological and non-pharmacological interventions will help to minimise any distress to the child.

