This article explores blood pressure (BP) in the context of stroke, regarding stroke severity and patient outcome. It discusses both ischaemic and haemorrhagic stroke.
Stroke is the second highest cause of mortality globally, resulting in 6.2 million deaths a year (Stroke Association, 2018). It also results in significant rates of disability; upon hospital discharge, two-thirds of stroke patients have a disability (Stroke Association, 2018). Because stroke has debilitating effects in both the short and long term, this pathological process is an important topic of discussion, and research into optimal care is vital to reduce the devastating impact on patients.
There are two key pathologies of stroke—acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH). They require different treatments, so initial management of stroke involves a rapid diagnosis of stroke pathology, via computed tomography (CT) angiography.
Patients experiencing AIS may be suitable for treatment with alteplase (Royal College of Physicians (RCP), 2016). Alteplase is a fibrinolytic agent, which produces plasmin via activation of plasminogen, resulting in thrombolysis (National Institute for Health and Care Excellence (NICE), 2018). To be eligible for treatment with thrombolysis, the patient's blood pressure (BP) must be below 185/110 mmHg. Therefore, treatment with antihypertensive therapy is recommended if the patient's BP is above this (RCP, 2016).
ICH is a less common stroke pathology, affecting 11% of patients (RCP, 2016). Initial treatment involves reversal of any anticoagulant therapy. Additionally, patients with a systolic BP above 150 mmHg should be treated with antihypertensive therapy to achieve a target systolic BP of 140 mmHg (RCP, 2016).
According to Appleton et al (2016), acute stroke impairs normal cerebral autoregulation. Cerebral autoregulation allows constant cerebral blood flow to be maintained despite changes in cerebral perfusion pressure (Hossman and Heiss, 2014). Following loss of functional cerebral autoregulation, systemic BP and subsequent cerebral perfusion pressure will alter cerebral blood flow. Therefore, excessively high or low BP will result in damage to brain tissue. Increased BP may lead to haemorrhagic transformation, cerebral oedema or haematoma expansion. Conversely, decreased BP may worsen tissue ischaemia (Appleton et al, 2016). Consequently, BP is an important aspect of stroke care and its management must be carefully considered to avoid worsening outcomes.
Multiple aspects of BP should be considered in both stroke pathologies. Literature surrounding BP in ischaemic and haemorrhagic stroke is discussed below, with a focus on the acute stages of stroke. Observations of BP and its management are examined to determine their impact on patients. These are discussed in relation to their effect on stroke severity and patient outcome in acute stroke. Better knowledge of BP in stroke will improve care, reduce stroke severity and ensure higher rates of positive patient outcome.
The research question was: how does blood pressure affect stroke severity and outcome in acute stroke patients?
Literature search
To produce the evidence required for discussion of the research question, a literature search was carried out. CINAHL Complete and MEDLINE Complete were searched, using the terms in Table 1. The research question could not be converted to the PICO (population, intervention, comparator and outcomes) format so no comparator was required. Boolean searching was performed.
| Group | Search terms |
|---|---|
| Group 1 (and) | Stroke |
| CVA | |
| ‘Cerebrovascular accident’ | |
| Group 2 (and) | ‘Blood pressure’ |
| BP | |
| Group 3 (and) | Outcome |
| Group 4 (and) | Emergency |
| Acute | |
| Group 5 (and) | Study |
| Studies | |
| Trial | |
| Group 5 (not) | Cardiovascular |
| Heart |
Search words in each group combined with AND/NOT/OR
Following the initial search, inclusion and exclusion criteria (Table 2) were applied to the articles found to generate a list of texts that might be relevant to the discussion. Finally, the full texts were analysed for their relevance to the research question. Ultimately, 15 articles were selected (Figure 1). This process was assisted by Zotero software, which enabled duplicate articles to be removed and texts organised easily.
| Inclusion criteria | Quantitative |
| Blood pressure | |
| Trial/study | |
| Primary research | |
| Stroke | |
| Maximum 15 years since publication | |
| Stroke | |
| Exclusion criteria | Analysis |
| Meta-analysis | |
| Protocol | |
| Qualitative | |
| Prevention |
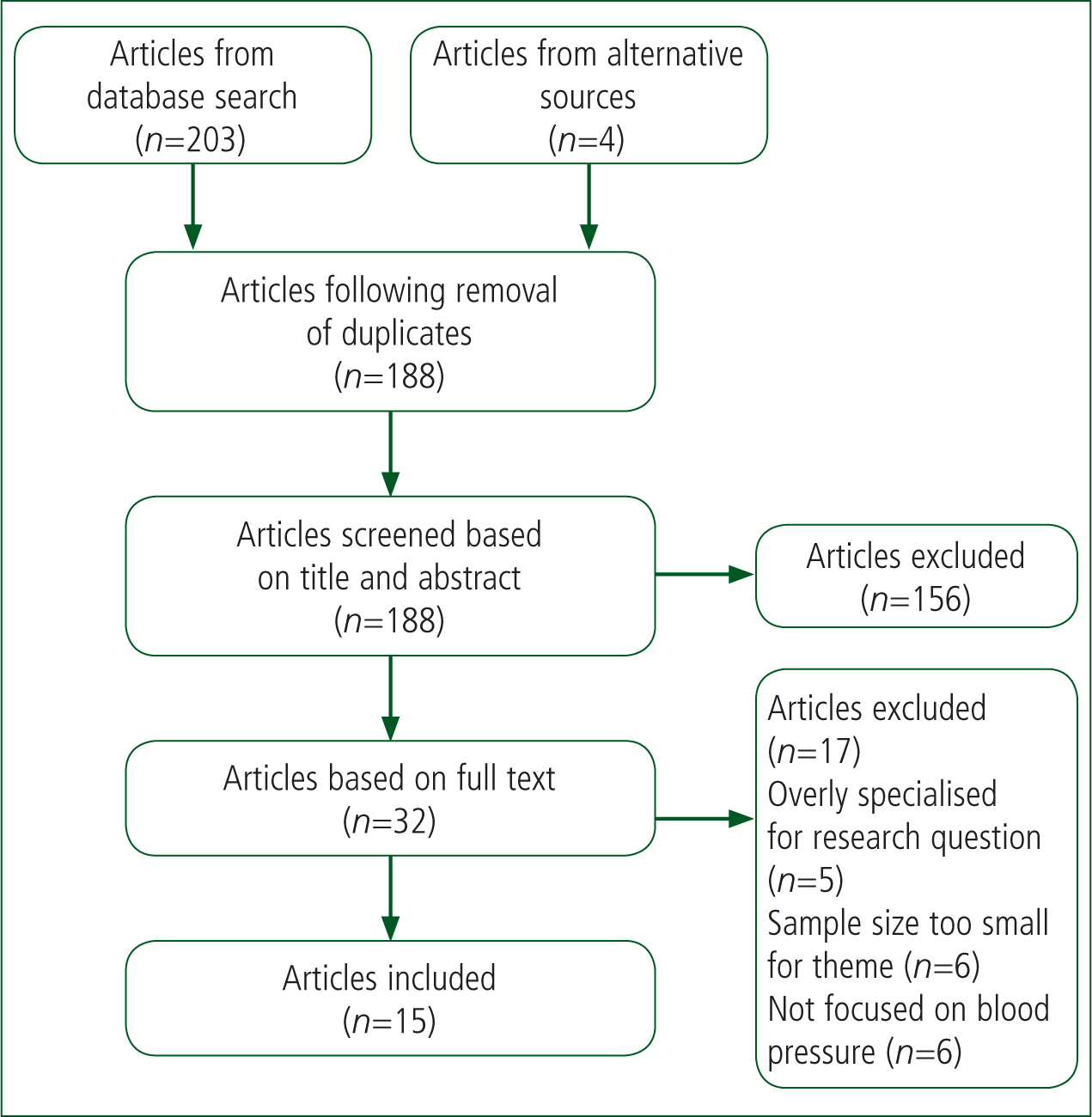
Quantitative research was selected as the most appropriate category of literature for answering the research question. The research question aimed to investigate correlations between variables in stroke, such as BP, stroke severity and outcomes. As these variables can be displayed numerically and analysed statistically, quantitative research is the most appropriate literature for answering this question (Cutter, 2012). The articles selected were critiqued using the Critical Appraisal Skills Programme (CASP) checklists for cohort studies and randomised controlled trials (RCTs) (CASP, 2018a; 2018b). Consequently, the studies could be categorised as providing strong or weak evidence.
The CINAHL Complete and MEDLINE Complete databases were used for the literature search. These were accessed via the library website at Anglia Ruskin University (where the author completed his degree in paramedic science) through a username and password-protected system.
The CINAHL database provides access to multiple nursing journals and therefore hospital-based studies (EBSCO Health, 2020a), so was a useful tool in generating relevant literature. The MEDLINE database provides access to health and biomedical journals and provided further relevant literature (EBSCO Health, 2020b).
Discussion
Admission blood pressure
In the 15 international articles found, admission BP is a prominent theme. Many of them aim to determine a correlation between BP on admission to hospital with various outcomes including death and disability.
Bentsen et al (2013) discuss the association of admission BP with patient outcome in the context of people with ischaemic stroke who were administered thrombolytic therapy. Patients were divided into four groups based on their admission BP. For each patient, this study recorded admission BP, National Institutes of Health Stroke Scale (NIHSS) score at admission and 24 hours post-thrombolysis, and Modified Rankin Scale (mRS) score at 3 months after thrombolysis (Bentsen et al, 2013).
The study found that the group with admission systolic BP in the 140–160 mmHg range had better outcomes than those with a BP above and below this range, while higher 24-hour NIHSS and 3-month mRS scores were associated with poorer outcomes. Furthermore, patients with lower BPs at admission had higher initial NIHSS scores, indicating higher severity strokes. There was a U-shaped correlation between admission BP and patient outcome (Bentsen et al, 2013).
Similarly, Kvistad et al (2013) investigated admission BP in patients with ischaemic stroke but focused more on stroke severity. Unlike Bentsen et al (2013), Kvistad et al (2013) included patients managed with thrombolysis or endovascular treatment and those who were not treated, so could analyse a wider range of patient subgroups with a larger sample size. Kvistad et al (2013) arguably produce higher-quality evidence than Bentsen et al (2013).
The patients in Kvistad et al's (2013) study were categorised into two groups according to BP on admission: non-elevated BP (below 140/90 mmHg); and elevated BP (above or equal to 140/90 mmHg). An inverse correlation was found between admission BP and stroke severity. There was a statistically significant association between non-elevated BP and severe stroke, defined as a NIHSS score ≥15. Furthermore, the study produced a statistically significant association between elevated BP and mild stroke, defined as an NIHSS score below 8 (Kvistad et al, 2013). These results are in line with those of Bentsen et al (2013), as lower admission BP is associated with higher stroke severity on admission. Kvistad et al (2013) suggest that this association is related to a protective response in ischaemic stroke, in which hypertension improves cerebral perfusion. They also argue that, if this hypothesis supported by the two studies is proven, this will provide evidence to support inducing hypertension in patients with ischaemic stroke.
Conversely, Kvistad et al (2013) were unable to determine a significant correlation between elevated admission BP and positive patient outcome. Furthermore, they found that elevated admission BP resulted in poor short and long-term outcomes, contradicting the hypothesis that the acute hypertension is protective and thus beneficial. However, only patients treated with thrombolysis were included in Bentsen et al's (2013) study, so the results may not be comparable.
Like Bentsen et al (2013), Wu et al (2016) investigated the relationship between initial BP and outcome in patients with ischaemic stroke treated with thrombolysis. BP was measured at baseline, at 2 hours and 24 hours after thrombolysis. Wu et al (2016) used a larger sample of patients within the same subgroup than Bentsen's et al (2013) study. Wu et al (2016) therefore had a more representative sample, which came from multiple hospitals, and overall higher-quality evidence than Bentsen's et al (2013) single-centre study.
Wu et al (2016) found an inverse correlation between systolic BP and favourable outcome at 90 days after stroke, defined as an mRS score of 0–1. They found that at a normal admission systolic BP of 140–160 mmHg, patients treated with thrombolytic therapy had better outcomes at 90 days after treatment than patients with hypertension. This finding is in agreement with Bentsen et al (2013).
Wu et al (2016) also found that patients with lower admission systolic BPs, namely below 140 mmHg, had better long-term outcomes than hypertensive patients. This finding contradicts Bentsen's et al (2013) study, in which systolic BP below 140 mmHg was associated with a poor outcome in both the short and long terms.
Yong et al (2007) explored the correlation between baseline BP and outcome in patients who had experienced an ischaemic stroke, with a focus on long-term outcome. They carried out a post-hoc analysis of an RCT in which participants received thrombolysis therapy or placebo. Yong et al (2007) found an S-shaped correlation between admission BP and favourable outcome (an mRS score of 0 or 1 at 90 days after thrombolysis) in both groups. In patients treated with thrombolysis, a systolic admission BP in the 155–165 mmHg range was associated with the highest rates of favourable outcome. Furthermore, in the 140–160 mmHg range, the proportion of patients with favourable long-term outcomes increased at a constant rate in both treatment and placebo groups (Yong et al, 2007). This suggests that a higher systolic BP is beneficial in terms of long-term outcome, up to a maximum of 160 mmHg, after which point the probability of favourable outcome decreases.
These findings are in agreement with those of Bentsen et al (2013), in that a systolic admission in the range 140–160 mmHg produces more favourable long-term outcomes. However, the findings from Yong et al (2007) show that a systolic BP towards the higher end of this range—160 mmHg—produces the highest probability of favourable outcome. Findings from Yong et al (2007) are also in agreement with those of Wu et al (2016), further reinforcing the benefits of systolic BP being in the 140–160 mmHg range.
Pezzini et al (2011) explored the relationship between BP and outcome in patients with both ischaemic and haemorrhagic stroke. BP was measured from admission continually for 48 hours and compared with various outcomes (Pezzini et al, 2011). Patients were categorised into two groups based on their average baseline systolic BP. In the low BP group, patients had a mean BP of 138 mmHg, with those in the high BP group having a mean BP of 171 mmHg. Pezzini et al (2011) found an association between a high baseline systolic BP and ICH, as a higher proportion of hypertensive patients presented with this pathology. Furthermore, the study found that early neurological deterioration was 15% more prevalent in the high BP group. In patients with ICH, the rate of unfavourable outcome at 90 days after a stroke was 28% greater in the high BP group (Pezzini et al, 2011). Pezzini et al (2011) argue that acutely elevated BP has a greater negative effect on outcome in ICH than in AIS. Although this study includes both ischaemic and haemorrhagic stroke patients, the sample size is low at 264 participants (Pezzini et al, 2011). The sample for ICH patients is particularly small, and may prove insufficient to produce statistically significant findings. Although the findings of this article may be considered, the evidence produced is weak.
Conclusions may be drawn regarding the effect of admission BP on clinical outcome in stroke. As the majority of the articles reviewed here focused on ischaemic stroke, the conclusions on this pathology have greater validity. A common finding of these studies is the association of an admission BP of 140–160 mmHg with positive short and long term outcomes in ischaemic stroke. This supports the management of acute ischaemic stroke by induction of hypertension to the BP range 140–160 mmHg. BP values above this range were commonly associated with poor outcomes, which provides evidence for maintaining BP below 160 mmHg. At BP values below 140 mmHg, findings were contradictory. From this, it may be deduced that the optimal range for BP in acute ischaemic stroke is 140–160 mmHg. This target BP range is especially beneficial in patients treated with thrombolytic therapy.
Blood pressure indexes and variability
Another prominent theme within these articles is BP indexes and BP variability. BP indexes, other than systolic and diastolic BP, include pulse pressure (PP) and mean arterial pressure (MAP). These aspects of BP shall be explored with regards to their relation to patient outcome.
Grabska et al (2009) investigated PP in patients with ischaemic stroke and the effect of this index on mRS score at discharge and death at 30 days after the stroke. They found that patients with an unfavourable outcome (mRS score ≥3) had a higher PP within 7 days of having had a stroke than those with a positive outcome. The mean PP for patients with favourable outcomes was 58.41 mmHg compared to 61.93 mmHg in those with unfavourable outcomes (Grabska et al, 2009: 189). A high PP was determined to be strongly associated with poor outcome at hospital discharge and mortality within 30 days of having a stroke (Grabska et al, 2009). Furthermore, an increase of 1 mmHg in PP resulted in a 1.3% greater mortality rate, also within 30 days of having a stroke. Grabska et al (2009) argue that these findings support reducing PP within 7 days of acute ischaemic stroke. Because of the large sample used in this study and consequent high external validity, the evidence is strong.
Kellert et al (2012) explored blood pressure variability (BPV) in ischaemic stroke patients treated with thrombolytic therapy and their outcomes, in particular, the development of ICH after thrombolysis. In this study, BP was measured continuously from admission until a follow-up scan within 36 hours of thrombolysis to produce values for BPV. Kellert et al (2012) found a correlation between high BPV and poor outcome, defined as an mRS score of ≥2 at 3 months after the stroke.
Kellert et al (2012) attribute this correlation to pathophysiological processes that result in poor outcome. First, they theorise that BPV in terms of an increase in BP may cause vessel rupture and consequent development of ICH or haemorrhagic transformation. Conversely, Kellert et al (2012) theorise that BPV in terms of a BP decrease would result in inadequate perfusion to the cerebral penumbra. However, they were unable to find an association of BPV with ICH following thrombolysis. Therefore it may be argued that BPV results in poor outcomes because of pathophysiological processes excluding ICH development.
Given the link between high BPV and poor outcome demonstrated by Kellert et al (2012), it may be relevant to argue against the use of antihypertensive medication in AIS because of its effect on BPV. Should BPV prove to have a greater link to outcome in stroke than admission BP, patients may benefit more from maintenance rather than the lowering of BP.
Bentsen et al (2013) measured BP changes from admission to 12 hours later. Their study found that the majority of patients experienced a decrease in BP between admission and 12 hours later. Patients with a systolic admission BP in the 140–160 mmHg range had a lower BP after 12 hours and could be considered normotensive, which was described as a ‘moderate’ decrease in BP (Bentsen et al, 2013: 2). These patients had better short- and long-term outcomes than those with admission BPs above 140–160 mmHg, who saw a greater decrease in BP between admission and 12 hours later (Bentsen et al, 2013). As a result, the poorer outcomes in this group may be attributed to the greater decrease in BP in the 12-hour time frame, rather than admission BP alone. This argument is in agreement with Kellert et al (2012), as greater change in BP results in poorer long-term outcomes. Bentsen et al (2013) also found that patients with an admission BP below 140 mmHg saw an ‘insignificant’ decrease in BP in the 12-hour time frame, and also had poorer outcomes than those in the 140–160 mmHg BP group. Although the change in BP was less significant, patients had poorer outcomes in this group, hence these findings contradict those of Kellert et al (2012).
These articles are difficult to compare because their methodologies differ. Bentsen et al (2013) examined values for BP at admission and 12 hours later, while Kellert et al (2012) calculated BPV based on 21 measurements for each patient. This allowed Kellert et al (2012) to produce a value that accounted for multiple oscillations in BP over the given time frame, so may be considered a more reliable tool for BPV measurement. Furthermore, Kellert et al (2012) used a larger sample than Bentsen et al (2013), so their findings may provide stronger evidence.
Zhang et al (2009) explored BP indexes and patient outcome in the context of haemorrhagic stroke. BP indexes, including MAP and PP, were calculated in the first 24 hours of admission. Zhang et al (2009) focused on short-term outcomes, namely death while in hospital and disability at hospital discharge. They found a positive correlation of systolic BP, diastolic BP, MAP and PP with death while in hospital. Furthermore, patients with elevated systolic BP, diastolic BP and MAP had higher dependency at hospital discharge, which was defined as an mRS score of ≥3 (Zhang et al, 2009).
Unlike Grabska et al (2009), Zhang et al (2009) did not find an association between PP and disability. Both of these studies used significant sample sizes so have high external validity. Grabska et al (2009) collected data from a single site, whereas Zhang et al (2009) collected data from multiple sites so had a more representative sample and consequently stronger evidence. However, the findings of these articles are difficult to compare because the patients investigated had differing pathologies. These findings suggest that elevated PP has a greater negative impact in ischaemic stroke than haemorrhagic stroke.
Active treatment of BP in acute stroke
The studies below investigated the treatment of BP and the consequent impact on stroke severity and patient outcome.
Darger et al (2015) reviewed patients with ischaemic stroke treated with thrombolysis and how their BP was being managed before this treatment. Darger et al (2015) investigated patients treated with aggressive, standard and no BP management strategies, and measured these against patient outcome. Patients receiving antihypertensive therapy were treated to reduce BP to below 185/100 mmHg to make them suitable for thrombolysis (Darger et al, 2015). Darger et al (2015) found that patients treated with aggressive BP therapy had the highest admission BP, at a median of 180 mmHg systolic, as well as the highest initial stroke severity, at a median NIHSS score of 13. These results are in conflict with the findings of Kvistad et al (2013), who demonstrated an inverse correlation between these two variables. However, Kvistad et al (2013) had a larger sample size so their findings have a higher external validity and therefore may be regarded as higher-quality evidence. Darger et al (2015) also demonstrated that patients with higher admission BP and NIHSS score are older, suggesting that age may have a significant impact on stroke severity.
In terms of mortality during hospitalisation, Darger et al (2015) did not find a significant difference between patients treated with antihypertensive therapy and those who were not before thrombolysis. The study found no significant difference in functional outcome at discharge, defined as an mRS score in the 0–2 range between patients requiring BP control and those who did not. The time from admission to treatment with thrombolytic therapy did not differ significantly between groups (Darger et al, 2015). These results suggest that controlling BP before thrombolysis is safe and does not reduce functional outcome rates on discharge. Therefore, from this study, it may be concluded that BP control will allow patients who initially are excessively hypertensive to be eligible for thrombolytic therapy with no increased risk of poor outcome.
He et al (2014) performed an RCT investigating the effect of antihypertensive therapy on outcome in ischaemic stroke, using a control group not given this treatment. Unlike Darger et al (2015), He et al (2014) included only patients not treated with thrombolytic therapy within 48 hours of stroke onset. He et al (2014) found that the mean BP of patients in the intervention group was significantly lower than that of those in the control group in the period from 4 hours to 14 days following group allocation. The intervention group had a mean average BP reduction of 21.8 mmHg systolic, compared to the control group's BP reduction of 12.7 mmHg in the first 24 hours (He et al, 2014).
He et al (2014) did not find a significant difference between the intervention and control groups in terms of death or disability, defined as an mRS score of ≥3, in the 14 days after randomisation or upon hospital discharge. They found no significant correlation between BP-lowering therapy and stroke severity measured by NIHSS score. Additionally, rates of mortality and disability were not significantly different within 3 months between the two groups (He et al, 2014). These results may be used to argue that active BP management is safe for patients with ischaemic stroke but not necessarily effective in improving clinical outcome. Therefore, in concordance with Darger et al (2015), BP management may be used to make hypertensive patients eligible for thrombolytic therapy through reducing BP below the recommended level of 185/110 mmHg.
Anderson et al (2013) performed an RCT comparing rapid BP reduction in hypertensive patients with ICH, with the standard guideline-recommended BP management strategy. This was the Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT2) (Anderson et al, 2013). Patients were randomised to one of two intervention groups. In the intensive group, patients received antihypertensive therapy to lower their systolic BP below 140 mmHg within 1 hour and maintaining this BP throughout 7 days of hospitalisation. The guideline-based group were treated with the aim of lowering BP below 180 mmHg (Anderson et al, 2013).
With regards to the primary outcome, death or an mRS score of 3–5 at 90 days, Anderson et al (2013) found no significant difference between the intensive and guideline-based treatment groups. However, they found a significant correlation between intensive BP management and overall better mRS scores at 90 days compared with the guideline-based management strategy.
In addition, Anderson et al (2013) used the European Quality of Life—5 Dimensions (EQ–5D) questionnaire. The results of these questionnaires showed that, at 90 days post randomisation, patients in the intensive management group had a better outcome in terms of quality of life than those treated using the guideline-based management strategy (Anderson et al, 2013).
Anderson et al (2013) argue that outcome is directly related to haematoma growth in ICH. The study provides data on a subgroup of patients in whom haematoma growth was measured for each intervention group. In both intervention groups, haematoma growth in a 24-hour period was not significantly different. Although intensive BP-lowering was associated with better long-term outcomes, haematoma growth did not differ between groups (Anderson et al, 2013). Therefore, it may be that a separate process resulted in better outcomes for the intensive group patients. Anderson et al (2013) attribute these better outcomes to rapid BP-lowering having a neuroprotective effect and reducing oedema.
Similarly, Qureshi et al (2016) randomised ICH patients into two groups including intensive treatment and standard treatment, in which patients were treated to achieve target systolic BPs below 140 mmHg and 180 mmHg respectively. Unlike Anderson et al (2013), Qureshi et al (2016) maintained this target BP for 24 hours instead of 7 days. The measured primary outcome was similar to that of Anderson et al (2013), being death or mRS score of 4–5 at 3 months after randomisation.
In concordance with Anderson et al (2013), Qureshi et al (2016) found no significant difference in the primary outcome between the two intervention groups. However, in contrast to Anderson et al (2013), Qureshi et al (2016) found no significant difference in the overall mRS scores at 3 months after randomisation between the two groups. Furthermore, using results from the EQ–5D questionnaire and comparing the intervention groups, there was no significant difference in quality of life at 3 months, further contradicting Anderson et al (2013) (Qureshi et al, 2016). In contrast to Anderson et al (2013), Qureshi et al (2016) found that patients in the intervention group had a greater rate of serious adverse events within 3 months. This suggests that intensive BP management in the first 24 hours of ICH may cause harm, although it does not affect 3-month neurological outcome or mortality.
The difference in results may be attributed to the time periods BP management was maintained by Anderson et al (2013) and Qureshi et al (2016). Anderson et al (2013) used intensive BP management for 7 days, and saw a better patient outcome than Qureshi et al (2016) who maintained BP management for 24 hours. From this, it may be argued that longer-term BP management following ICH results in better patient outcome. While both of these studies are RCTs, Anderson et al (2013) analysed a far larger sample, so their study has higher external validity and arguably stronger evidence for this discussion.
The ENOS Trial Investigators (2015) performed an RCT to determine the effect of transdermal nitric oxide therapy on BP and the subsequent outcome in both ischaemic and haemorrhagic stroke with hypertension. Patients were randomised to receive transdermal glyceryl trinitrate (GTN) patches or a placebo within 48 hours of symptom onset. The ENOS Trial Investigators (2015) found no significant difference in outcome, assessed using mRS and quality-of-life measures, between both groups at 90 days. The GTN group had an average BP that was lower than that of the control group in only the first 3 days of treatment. After 3 days, the difference in BP was insignificant. Additionally, the reduction of BP in the two groups following the first dose was 7 mmHg for the GTN group and 3.5 mmHg for the control group (ENOS Trial Investigators, 2015). It could be argued that the antihypertensive effect of GTN patches was not sufficient to produce a significant BP decrease and consequently better outcomes.
Like Anderson et al (2013), the ENOS Trial Investigators (2015) continued antihypertensive treatment for 7 days. However, unlike Anderson et al (2013), the ENOS Trial Investigators (2015) found no improvements in any of the outcome measures between the two groups. This may be attributed to the smaller BP decrease they saw compared to that demonstrated by Anderson et al (2013). From this, it may be suggested that more intensive BP management, which produces a sharper decrease in BP, is beneficial in terms of outcome. However, Anderson et al (2013) focused solely on haemorrhagic stroke, compared to the ENOS Trial Investigators (2015), who investigated both pathologies. Additionally, both studies use different pharmacological agents to produce BP change. Therefore, it may be difficult to compare the results directly.
Prehospital and primary care
The Joint Royal Colleges Ambulance Liaison Committee (JRCALC) guidelines outline the recommended management plan for patients with suspected stroke/transient ischaemic attack within UK ambulance services (Association of Ambulance Chief Executives (AACE, 2019). The guidelines highlight the importance of initial ABCD (airway, breathing, circulation and disability) assessment and the FAST (facial drooping, arm weakness, speech difficulties and time-to-call emergency services) test, as well as eliminating differential diagnoses such as hypoglycaemia. Furthermore, there is an emphasis on reducing delays on scene and providing rapid transport to hospital or a hyperacute stroke unit if available (AACE, 2019).
So far, the articles discussed have focused on in-hospital observation or treatment of BP in stroke. The three articles below concern prehospital BP management in stroke.
Ankolekar et al (2013) performed an RCT based in one UK ambulance service to determine the feasibility of a prehospital trial and the efficacy of the BP management strategy. Paramedics randomised stroke patients to receive a transdermal GTN patch or placebo, which was continued for 6 days after randomisation in hospital (Ankolekar et al, 2013). The GTN group experienced a significantly greater decrease in BP than the control group, of 21 mmHg within 15 minutes of treatment starting (Ankolekar et al, 2013). Using the Scandinavian Stroke Scale (specific to this study) to measure disability, Ankolekar et al (2013) determined that at 7 days after randomisation, there was no significant difference in Scandinavian Stroke Scale scores, rate of mortality, adverse events or deterioration. However, in terms of 90-day outcome measured using mRS, patients in the GTN group had significantly better outcomes than those in the control group (Ankolekar et al, 2013).
Both Ankolekar et al (2013) and the ENOS Trial Investigators (2015) used transdermal GTN for BP management in both ischaemic and haemorrhagic stroke. In these studies, treatment was continued for 6–7 days with equal doses of GTN, but Ankolekar et al (2013) demonstrated that patients treated with GTN had better 90-day outcomes in terms of mRS scores. In comparison, no such benefit was seen in the study by the ENOS Trial Investigators (2015).
The disparity in results may be attributed to difference in time from stroke onset to treatment between the studies. Ankolekar et al (2013) gave treatment in the prehospital environment within an average of 55 minutes of stroke onset compared to an average of 26 hours in the study by the ENOS Trial Investigators (2015). Consequently, it could be concluded that starting BP management early is beneficial in producing functional long-term outcomes in both pathologies of stroke. Conversely, given the greater BP decrease produced by Ankolekar et al (2013) compared to that by ENOS Trial Investigators (2015), it could also be debated that more intensive BP management is beneficial in producing better long-term outcomes. However, despite this potential conclusion, Ankolekar et al (2013) had a small sample, so their study has low external validity and is weaker evidence than that by the ENOS Trial Investigators (2015).
In contrast to the previous study, that by the RIGHT-2 Investigators (2019) included 1149 participants, of whom 568 were treated with transdermal GTN and 581 had a placebo dressing. The large sample size produces stronger evidence than the previous study, although the results of the RIGHT-2 study were less significant. The RIGHT-2 Investigators (2019) found no clinical benefit in the use of transdermal GTN in stroke patients with regards to outcomes, measured using mRS. Furthermore, transdermal GTN produced a systolic BP decrease of only 5.8 mmHg, a significantly lower value than that in the RIGHT trial, which found a decrease of 21 mmHg (Ankolekar et al, 2013). This further supports the argument that more intensive BP management produces better outcomes for patients who have had a stroke.
Grabska et al (2013) explored the effect of pre-stroke antihypertensive treatment, administered in a primary care setting, on patient outcome in ischaemic stroke. Patients treated with antihypertensive therapy before they experienced a stroke had lower admission BP than those not treated before their stroke. Having pre-stroke antihypertensive therapy did not significantly affect mortality within 30 days of onset (Grabska et al, 2013). However, at 30 days after the stroke, 38.4% of patients treated before the event had ‘excellent outcomes’, defined as an mRS score <1, compared with 29.6% of untreated patients (Grabska et al, 2013: 144). The researchers suggested that antihypertensive therapy before a stroke was a ‘significant predictor of stroke outcome’ (Grabska et al, 2013: 144). These results further support findings by Ankolekar et al (2013) that early initiation of BP management produces better outcomes, even before stroke onset.
Recommendations
The studies in the present review identified a number of themes that confirm the importance of BP and its management in acute stroke.
It may be concluded that an admission systolic BP in the 140–160 mmHg range is associated with a better outcome. Therefore, in the acute stages of stroke, it may be beneficial to treat BP to bring it within this range. This may mean a compromise between adequate cerebral perfusion pressure and preventing cerebral oedema. In ischaemic stroke, BP management is safe and should be used to increase eligibility for thrombolysis. NICE (2018) guidelines recommend antihypertensive treatment in BP values above 180/105 mmHg when using alteplase for AIS (NICE, 2020). This discussion may provide an argument for reducing BP to 140–160 mmHg systolic BP in the acute phase of stroke.
In AIS, patients with a PP above normal values have a higher probability of poor outcome in the short term. Furthermore, patients with AIS whose BP decreases within 12 hours of admission have better outcomes. In ICH, elevated indexes including diastolic, systolic, MAP and PP result in higher rates of mortality, although PP is not associated with impairment in this pathology.
Following the stroke pathology diagnosis, patients with ICH should be provided with intensive BP-lowering therapy to a target of 140 mmHg and this level maintained for a minimum of 7 days, as adopted into the National Stroke Guidelines (RCP, 2016). During hospitalisation, fluctuations in BP should be avoided as high BPV leads to a poor outcome. In the prehospital environment, starting BP management early is beneficial to patients, and this finding shows that further research into prehospital stroke care is required.
Systolic BP above 140 mmHg is associated with milder strokes and systolic BP below 140 mmHg with higher NIHSS scores and therefore greater stroke severity. Conversely, despite higher stroke severity, the evidence on outcome in patients with systolic BP below 140 mmHg was contradictory. Further research into admission BP may be necessary, especially regarding values below 140 mmHg.
From a prehospital perspective, where making a diagnosis of stroke pathology is generally not possible, these results provide evidence for the provision of antihypertensive therapy to patients experiencing a stroke to achieve a target BP of 140 mmHg. This lies within the beneficial range of 140–160 mmHg for patients with AIS, as well as the beneficial target BP for ICH. Therefore, adopting this target BP in the prehospital environment will lead to higher rates of thrombolysis eligibility for AIS patients and better outcomes for those with ICH, despite the inability to diagnose stroke pathology.
This discussion included articles from 2008 to 2019. The guidelines regarding thrombolysis treatment changed during this period; the recommended time window in which patients should be treated with thrombolysis widened in 2012 from 3 to 4.5 hours from initial stroke onset (RCP, 2016).

