LEARNING OUTCOMES
After completing this module the paramedic will be able to:
If you would like to send feedback, please email jpp@markallengroup.com
Infections of the heart can result in significant harm and includes cardiac tamponade, congestive heart failure, emboli, dilated cardiomyopathy (DCM) and death (McDonald, 2009; Blauwet and Cooper, 2010; Curry, 2014). These infections can be broken down into three main areas that correspond with the layers of the heart: endocarditis (endocardium), myocarditis (myocardium) and pericarditis (pericardium). Out of the three, the most common is pericarditis, which accounts for 5% of all chest pains that are presented to emergency departments (Curry, 2014). Forms of pericarditis are included in the differential diagnosis algorithm for chest pain that is documented by Naumov (2009). It is also included by Snyder et al (2012) as one of the causes of non-acute coronary syndrome (non-ACS). These indicate its prevalence enough to be considered as a relatively common cause of chest pain. Although reported cases of myocarditis are not as high as pericarditis, it is a disease that can cause life-threatening complications. It is mostly notable in the paediatric patient, where it accounts for 12% of sudden cardiac death among adolescents and young adults (Levine et al, 2010). Endocarditis has a high mortality rate for those who contract it (Weymann et al, 2014). In the general population, the incidence of endocarditis is low (1.7–6.2 cases per 100 000 patients). This rises significantly in intravenous drug users (IVDU) to 2–5% and is responsible for 5–10% of deaths in this patient group (Weymann et al, 2014).
As ACS remains a leading cause of preventable early deaths (Figgis et al, 2010), emphasis is placed on this condition, with less emphasis on other causes such as cardiac infections (Snyder et al, 2012). This module examines the three main groups of cardiac infections individually. It then discusses whether pre-hospital diagnosis by the ambulance service could improve the care for patients presenting with a differential diagnosis of one of the cardiac infections.
Pericarditis in relation to chest pain
Pericarditis is the inflammation of the pericardium around the heart. Pericarditis can be broken down into two major groups that cause this disease: infectious (accounting for two thirds of the incidents) and non-infectious (Imazio et al, 2010). Table 1 (Imazio et al, 2010) shows how the two main groups are classified.
| Aetiology of pericarditis: a simple basic classification is infectious (more than two of three cases) and non-infectious forms (percentages refer to unselected cases) |
| Infectious pericarditis (2⁄3 of cases): |
| Major causes of recurrences still remain idiopathic, others include viral, autoimmune forms, inadequate treatment of the index attack (in relation to doses and⁄or length of therapy) and neoplasia. EBV, Epstein-Barr virus; CMV, cytomegalo virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
Pericarditis is associated with sharp retrosternal pain (98% of cases) that may radiate to the neck, shoulders or arms. Other symptoms include pericardial rub (35% of cases), a fever of 38°C or more, and signs of cardiac tamponade. An ECG would commonly show diffuse ST elevation and PR depression in all leads except AVR and V1. They can also show reciprocal ST depression and PR elevation (Rahman and Liu, 2011), as shown in Figure 1 (Curry, 2014)
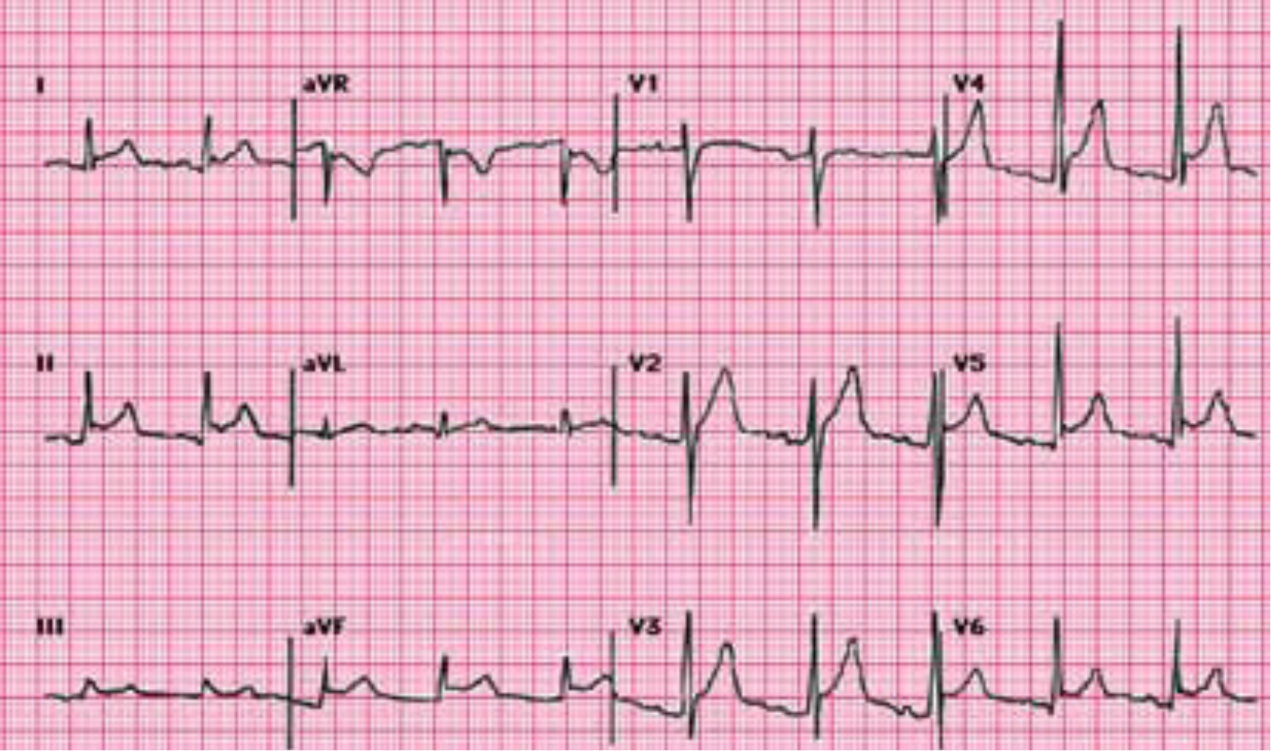
Complications of pericarditis include pericardial effusion (present in 60% of cases), myopericarditis, recurrent pericarditis and tamponade (Rahman and Liu, 2011). Constrictive pericarditis is also a life changing possible complication of pericarditis (Imazio et al, 2011). Although the risk of the more dangerous complications are low with idiopathic or viral pericarditis (most cases of pericarditis are classed as idiopathic as the process of testing for specific causes is not productive in the treatment for the majority of cases), bacterial pericarditis—which is not common in the western parts of the world—has a higher risk of complications and death if it is not treated (Curry, 2014). Imazio et al (2011) looked specifically at complications that occur with pericarditis. The results of this study showed that for idiopathic or viral pericarditis, constrictive pericarditis had an incidence of 0.48% (n=416) of the cases. For bacterial causes, the incidence rates varied between 2.8% (n=36) for connective tissue disease and 33% (n=3) for purulent pericarditis. This study looked at 500 cases from January 2000 to December 2008. Out of the 500 patients with acute pericarditis, only nine showed clinical signs of constrictive pericarditis. Seven of these were due to non-viral/non-idiopathic aetiology. Non-viral/non-idiopathic aetiology accounted for 87 of the 500 cases. These results had a p value of <0.001 and so can be regarded as significant. The study also investigated the complications of cardiac tamponade (4.4% of cases) and recurrent pericarditis (30% of cases). The results showed a trend when idiopathic/viral pericarditis was compared to specific aetiology. Complications for specific aetiology pericarditis were always more prevalent than for idiopathic/viral pericarditis.
The mainstay of treatment for pericarditis includes non-steroidal anti-inflammatory drugs (NSAIDs), colchicines (Rahman and Liu, 2011) and corticosteroids (Curry, 2014). Patients must be assessed for risk factors that could contribute towards complications. These include high fever, subacute severe pericardial effusion, cardiac tamponade and lack of response to NSAIDs (Imazio et al, 2010). Dependent on the risk factor of the patient, admission or discharge will then be considered, as well as treatment for underlying causes. Education of pericarditis for the patient is also recommended (Buckley and Cabrera, 2013).
Myocarditis and the paediatric patient
Myocarditis had an inversely logarithmic association with age in a study performed in Finland on patients aged over 16 years (n=3198 myocarditis patients, r=-0.97, p<0.0001) (Kytö et al, 2013). Although myocarditis happens most commonly in the younger aged patient, it is still an uncommon paediatric and young adult illness (Blauwet and Cooper, 2010; Levine et al, 2010). Myocarditis accounts for 12% of sudden cardiac death among children and young adults (Levine et al, 2010). Studies have also shown mortality of the disease carries on years after the initial onset. Blauwet and Cooper (2010) suggested children could still be at risk of death or complications such as dilated cardiomyopathy (DCM) that require cardiac transplantation up to 12 years after diagnosis. As myocarditis is a relatively uncommon disease, for researchers to gain a large enough sample of patients, the retrospective studies generally need to date back a number of years. When this is taken into account along with improvements in the diagnosis, awareness and treatment of myocarditis, the prognosis of myocarditis could be better than what the research suggests.
As with pericarditis, there are many causes of myocarditis with infections being a major cause. Table 2 (Elamm et al, 2012) shows these categories.
| Viruses/disorders | Baceria/disorders | Cardiotoxins | Hypersensitivity |
|---|---|---|---|
| Adenovirus* | Chlamydia | Ethanol* | Cephalosporins |
| Coxsackievirus B* | Cholera | Anthracycline drugs* | Clozapine |
| Cytomegalovirus* | Mycoplasma | Arsenic | Diuretics |
| Epstein–Barr virus | Neisseria | Carbon monoxide | Insect bites |
| Hepatitis C virus | Salmonella | Catecholamines | Lithium |
| Herpes simplex virus | Staphylococcus | Cocaine* | Snake bites |
| HIV* | Streptococcus | Heavy metals | Sulfonamides |
| Influenza virus | Tetanus | Copper | Tetanus toxoid |
| Mumps | Tuberculosis | Mercury | Tetracycline |
| Parvovirus B19 | Spirochetal | Lead | Systemic disorders |
| Poliovirus | Leptospirosis | Protozoa | Hypereosinophilia |
| Rabies | Lyme disease | Chagas disease | Kawasaki disease |
| Rubella | Relapsing fever | Leishmaniasis | Sarcoidosis |
| Varicella zoster virus | Syphilis | Malaria | Wegener granulomatosis |
| Yellow fever |
The main issue with myocarditis is the variety of symptoms. Most of the symptoms are seen in common ailments such as fever, shortness of breath, and upper respiratory infections. Shu-Ling et al (2013) performed a retrospective review into the initial diagnosis of paediatrics with subsequent confirmed myocarditis. They found that 38.5% of cases (n=38) were correctly diagnosed by emergency physicians with the most common mis-diagnosis being respiratory tract infections (20.5%). Durani et al (2009) performed a retrospective study to investigate signs and symptoms that were present in 62 paediatric patients that were diagnosed with either myocarditis or DCM. The results are shown in Box 1 and show similar results to Shu-Ling et al (2013), in that the variety of presentations of myocarditis is vast.
In hospital there are a number of specific tests to diagnose myocarditis. Non-invasive procedures include cardiovascular magnetic resonance imaging (CMR), which is the leading modality for non-invasive imaging (Childs and Freidrich, 2011). The gold standard, however, is endomyocardial biopsy. The problems with this procedure are that it is invasive and most clinical settings have limited ability to perform it (Elamm et al, 2012). In the pre-hospital setting there are a lot fewer tests that can be performed. Clinicians need to have a high level of suspicion of myocarditis. Clinicians working in the emergency setting have to attend and diagnose a wide variety of conditions which leaves less common conditions such as myocarditis less likely to be considered. As it has been shown, the symptoms that are presented by myocarditis are varied. Shu-Ling et al (2013) concluded their research commenting that any child with symptoms of hypoperfusion—especially unexplained seizures or syncope—should have an ECG performed. Findings on an ECG can also vary widely. The most common ECG finding is non-specific T-wave changes. The ECG can also show up ST-segment elevation or depression, PR depression and pathological Q waves (Elamm et al, 2012). Elamm et al (2012) state that ECGs have a very low sensitivity for diagnosing myocarditis. This is supported by Blauwet and Cooper (2010), who put the sensitivity of ECGs in myocarditis at 47%. Although diagnosis through ECGs is not very sensitive, the findings by Durani et al (2009) concluded that all of the 62 patients with myocarditis or DCM had ECG changes. Shu-Ling et al (2013) supported this finding with their own results that found all 39 of their patients diagnosed with myocarditis over a 10-year period showed ECG changes. This would suggest that if, in the pre-hospital environment, a paediatric patient presented with an abnormal ECG and had signs of hypoperfusion, myocarditis should be part of the differential diagnosis. The problem with this conclusion is that paramedics are not trained on ECGs in paediatrics and would rarely, if ever, perform a diagnostic 12-lead ECG on a paediatric patient. The difficulties of interpretation of paediatric ECGs were highlighted in a study performed by Jheeta et al (2014). Of the 493 UK paediatricians registered with the UK Royal College of Paediatricians and Child Health that took part in the study, only 61.5% correctly identified the 10 ECGs before more training was given.
The main complication with myocarditis is DCM. DCM is associated with systolic dysfunction and can cause left, right or biventricular failure. It is associated with sudden cardiac death and heart failure (Jeffries and Towbin, 2010). The mechanism from myocarditis to DCM typically takes three stages. The first stage is the injury and activation of innate immunity. This leads to the second stage, which is acute myocardial inflammation. This then progresses to the third stage, which is DCM (Elamm et al, 2012). Figure 2 (Blauwet and Cooper, 2010) shows this progression.
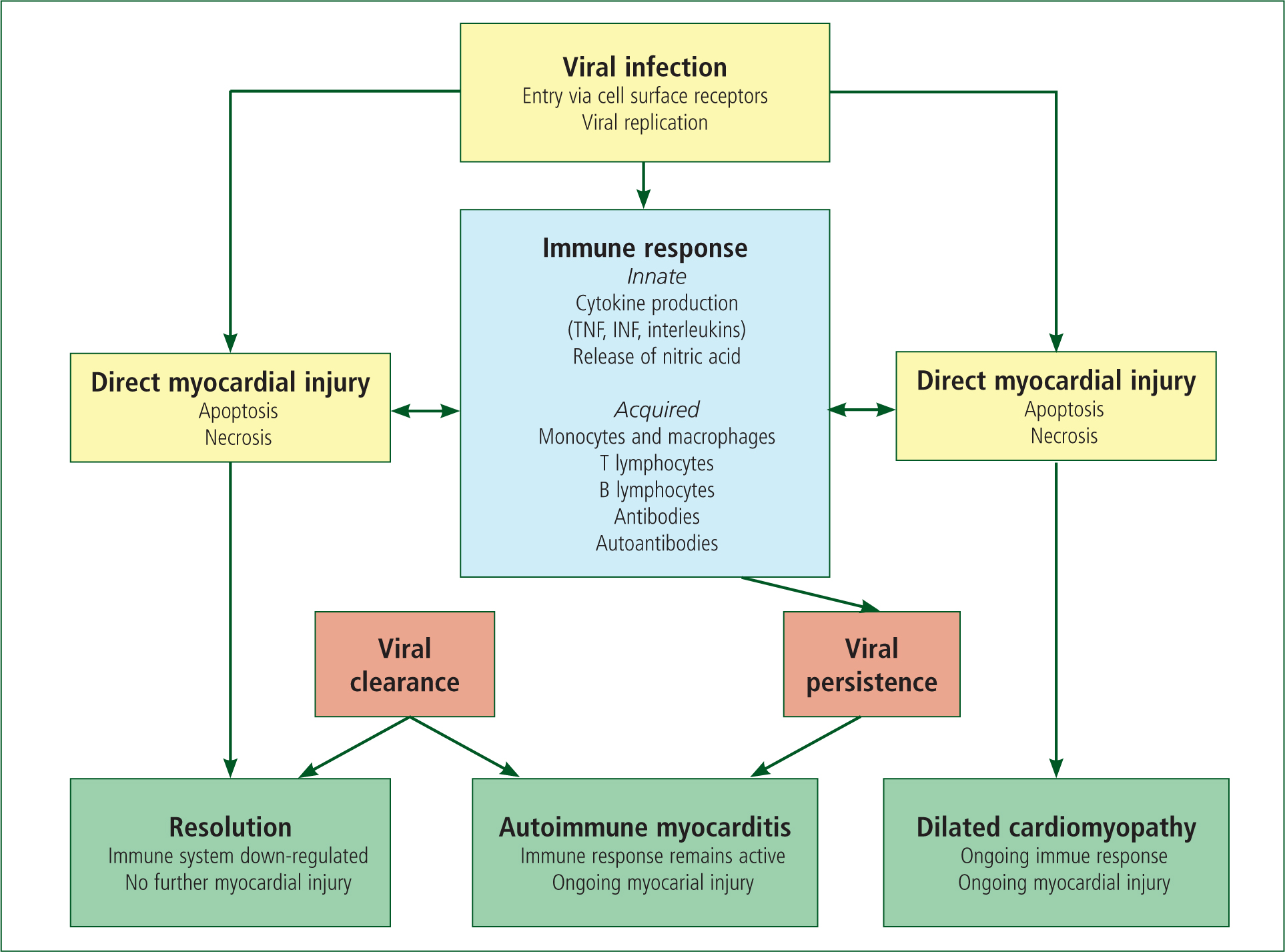
In most patients, the pathogen is cleared and the immune reaction is down-regulated with no further adverse effects. For some patients the virus is not cleared, causing the myocardial inflammation due to the immune response to cardiac autoantibodies (Blauwet and Cooper, 2010). The prognosis of patients depends on important variables. These include the degree of left and right ventricular dysfunction, heart block and specific histopathological forms of myocarditis (Schultz et al, 2009).
Treatment for myocarditis depends on the presentation. The cornerstone of any therapeutic approach is to treat the heart failure or arrhythmia (Kühl and Schultheiss, 2012). Blauwet and Cooper (2010) researched individual drug therapies. Patients with signs of DCM responded well to heart failure therapy. NSAIDs were not effective in several models that involved mice and could result in increased inflammation and higher mortality rate. Antiviral treatment created mixed results. This was supported by Kühl and Schulthesis (2012). Blauwet and Cooper (2010) hypothesised that this could be due to a lot of patients with myocarditis being diagnosed weeks after the viral infection. Immunosuppressive treatment was not very effective in most cases but did have good results for patients with giant-cell myocarditis. Intravenous immunoglobulin led to improved recovery of left ventricular function in children but had very little effect on adults. Blauwet and Cooper (2010) also found that exercise should be avoided for the first 6 months as it leads to increased mortality.
Endocarditis and the drug user
In myocarditis, the incidence rates are the highest in the younger patient. The opposite trend is found in endocarditis. The incidence rate of infective endocarditis (IE) in the general population is between 2 and 10 per 100 000 people (McDonald, 2009). In the elderly it is as high as 20 per 100 000 people. Although these are still low figures, IE accounts for 1 in 1 000 hospital admissions in the US (McDonald, 2009).
Incidences of IE have increased with the improvement of clinical procedures. It is a complication particularly with procedures such as the implanting of cardiac implantable electronic devices (McDonald, 2009; Kim et al, 2014; Thanavaro and Nixon, 2014). Kim et al (2014) also found that the mortality rate of the patients who contracted IE due to the implants was 36% (n=80). Other research also showed high mortality rates in patients who have been diagnosed with IE. Damasco et al (2014) found a mortality rate of 46.6% (n=71). McDonald (2009) found the mortality rate to be 20–30% with certain causes of IE. IE caused by gram-negative bacilli and fungi had a mortality rate of over 50%.
The mechanism that causes endocarditis starts with injury of the endocardium. The endocardial damage then triggers sterile thrombus formation. Once a thrombus is present, transient bactereremia can seed the thrombus. Bacteria in the blood stream can also adhere to the thrombus. Once bacteria has attached to the endocardium, the vegetation matures (McDonald, 2009).
In drug users, the cause of the endocardial injury is the direct injection of contaminated debris. This causes damage to the right sided valves and is the majority of the causes of right sided IE (McDonald, 2009).
The diagnosis of IE, as in myocarditis, requires a high index of suspicion due to the range of non-specific symptoms that are associated with the diagnosis. The Modified Duke Criteria has become the gold standard for the diagnosis of IE (Thanavaro and Nixon, 2014) (See Box 2).
Although the Modified Duke Criteria is the gold standard for diagnosis, this is for in-hospital diagnosing. For the pre-hospital environment, the facilities that are available do not allow this criterion to be followed and so IE needs to be part of a differential diagnosis based on the patient's symptoms. The main tool available to the pre-hospital clinician is the history and clinical examination. Symptoms of endocarditis include fevers, chills, fatigue, malaise and weight loss (Thanavaro and Nixon, 2014). Other symptoms include symptoms of an embolism or heart failure. On physical examination, 85% of patients with IE present with a heart murmur. A history examination should look at the high-risk factors such as invasive procedures, prior endocarditis, structural heart disease and injection drug user (McDonald, 2009).
Rodriguez et al (2011) performed a retrospective study into intravenous drug users (IVDU). The study looked at the possibility of a tool that can categorise IVDU presenting to emergency departments with fever from an unclear source. The study included three clinical findings to categorise whether a patient is a low risk for endocarditis or not (See Figure 3).
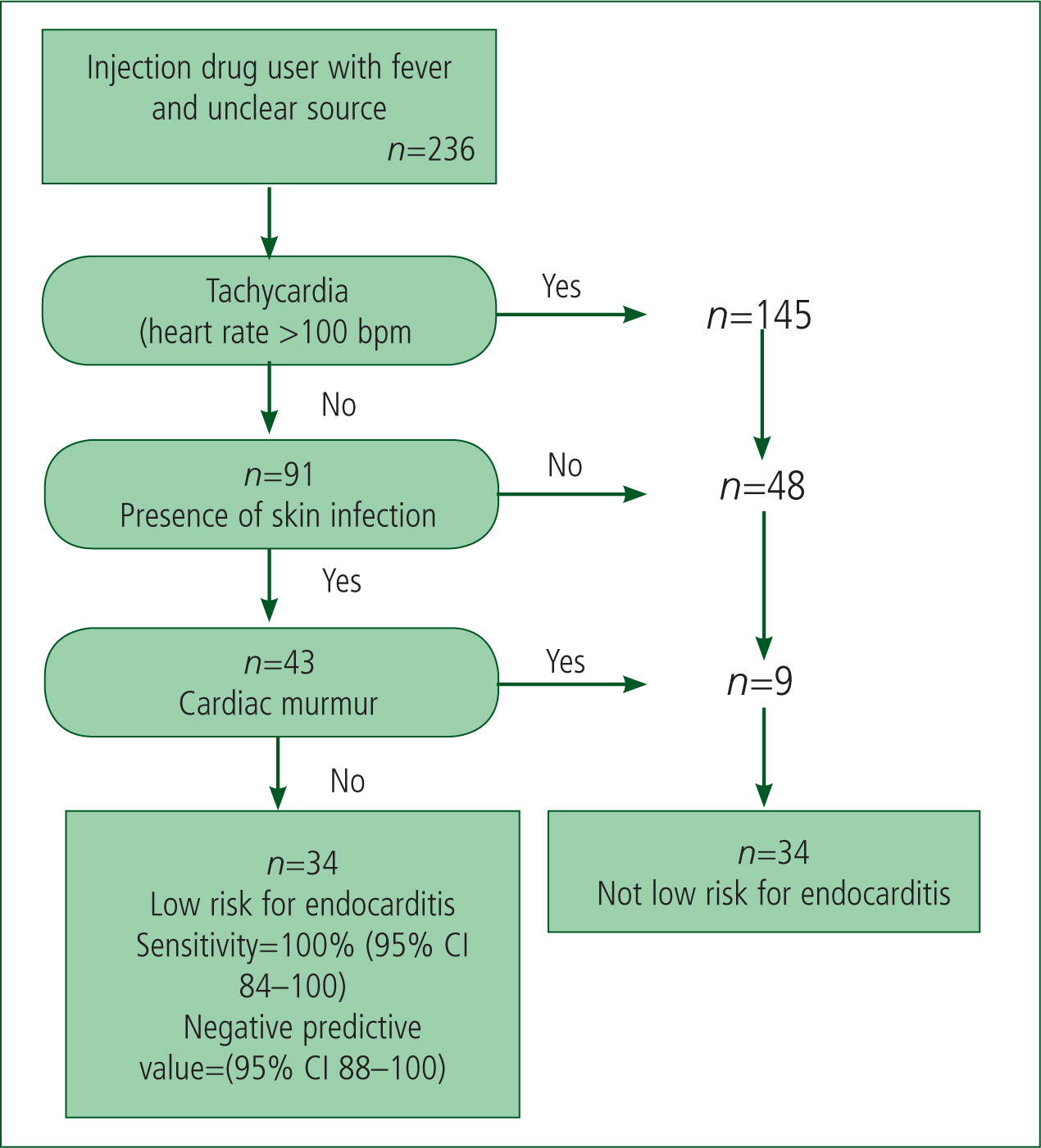
As Figure 3 highlights, if this tool was used with the 236 patients that were included in the retrospective study, 34 patients would not have needed more expensive tests performed to exclude endocarditis. Out of the 202 who were categorised as not low risk, all of the 20 subjects who were diagnosed with endocarditis were present. The tool is safe in the fact that no diagnosed endocarditis was in the low-risk category. Although the tool still put 182 patients not diagnosed with endocarditis in the ‘not low risk’ group, the clinical presentations are low cost and can be done pre-hospital.
Chung-Esaki et al (2014) followed up this study with clinical data on 249 subjects from 2007 to 2011. In total, 29 patients were identified as low risk for endocarditis. All 18 patients who were diagnosed with endocarditis were in the ‘not low risk’ group. Chung-Esaki et al (2014) documented that this tool, should it be externally validated, could be used to rule out endocarditis in IVDU in a similar manner to the Ottawa Ankle and NEXUS Cervical Spine rules.
Aggressive and early treatment with antibiotics is the cornerstone for treatment of endocarditis. Sometimes there is a possibility of the necessity of surgery (McDonald, 2009; Damasco et al, 2014; Thanavaro and Nixon, 2014).
Häällgren and Lindqvist (2010) reviewed the medical records of three IVDUs diagnosed with endocarditis. It found that two of the cases bought their drugs from the same drug dealer whose hygiene standards were poor and so risked the chances of faecal contamination of the drugs. Aggressive antibiotics worked for one case but antibiotics and surgery was needed for the other. This highlights another form of history taking in that when a IVDU is showing signs of endocarditis, the medical history of friends who use the same drugs distributor might assist in the diagnosis of endocarditis. The additional advantage is if the first patient has responded well to an antibiotic, the antibiotic might also be the best one to use for the second patient if the bacterium is from the same source.
Weymann et al (2014) reviewed surgical treatment on 20 cases of IVDU endocarditis. These patients were severe cases and the mortality rate for these patients would have been very high without surgery. With surgery, the survival rates for these patients at 1, 5, and 10 years were 90%, 85% and 85%, respectively, without any need to reoperate. Although a larger sample would be required to support the study by Weymann et al (2014), these statistics show that surgery is an effective procedure for IVDUs that are diagnosed with endocarditis and show signs of significant complications.
Discussion
Pericarditis is a relatively common cause of chest pain and should, at the start of the consultation, be part of the differential diagnosis of patients with chest pain. The diagnosis and treatment of acute life-threatening diseases, such as myocardial infarctions, is the priority. This should not be to the exclusion of all other possible conditions. If there is evidence pericarditis is the most likely cause of the chest pain, the paramedic would show more professionalism if this was documented. The treatment of the patients as per acute myocardial infarction protocols includes aspirin, nitrate and pain relief. The treatment of pericarditis includes NSAIDs and pain relief. If a clinician was to treat a patient with pericarditis in the same way as a patient with acute myocardial infarction, the initial therapy would not be too dissimilar. In a scenario where the paramedic is not confident to fully diagnose pericarditis but has a suspicion of it, to give the treatment for myocardial infarction but also document pericarditis would ensure that the patient is treated for the disease with higher mortality. This would also not have a negative effect on the most likely cause. The contribution towards the patient's treatment would be that the differential diagnosis of pericarditis would have been highlighted to hospital staff. Initial tests for pericarditis could then be started at an earlier stage in the patient's treatment if the hospital staff deems this to be appropriate.
The contribution towards the paramedic profession is that the paramedic shows more professionalism by demonstrating that they can use a broader knowledge in chest pain. Naumov (2009) listed 43 possible causes of chest pain (Table 5). The paramedics who are able to consider more of the conditions listed by Naumov (2009) will demonstrate a larger knowledge base. Demonstration of the ability to perform good differential diagnosis will also assist in the future development of the paramedic role. One future development is a prescribing role that was proposed by the National Ambulance Service Medical Directors (2014). Being able to get an informed diagnosis from a broad knowledge of differential diagnoses is important for this role.
| Cardiovascular diseases | Pulmonary and pleural diseases | Abdominal dieseases | Muskuloskeltal disorders | Miscellaneous disorders |
|---|---|---|---|---|
| Acute myocardial infarction | Acute massive pulmonary embolism | Carcinoma of oesophagus | Myalgia | Herpes zoster |
| Aortic dissection | Pulmonary thromboembolism | Diffuse oesophageal spasm | Cervical osteochondrosis | Familial Mediterranean fever |
| Stable angina pectoris | Pulmonary infarction | Reflux oesophagitis | Intercostal neuralgia | Cystic fibrosis (adult form) |
| Unstable angina pectoris | Staphylococcal pneumonia | Subphrenic abscess | Tietze's syndrome | Anxiety neurosis |
| Crescendo angina | Pneumococcal pneumonia | Acute pancreatitis | Traumatic rib fracture | |
| Angina variant (Prinzmetal's) | Klebsiella pneumonia | Irritable bowel syndrome | Pathological rib fracture | |
| Acute non-suppurative pericarditis | Pneumothorax | Traumatic pneumothorax | ||
| Acute suppurative pericarditis | Primary pulmonary hypertension | Traumatic haemothorax | ||
| Cardiac tamponade | Secondary pulmonary hypertension | |||
| Aortic valvular stenosis | Pulmonary abscess | |||
| Cardiomyopathy secondary | Atelectasis | |||
| Patent ductus arteriosus with right to left shunt | Bronchial adenocarcinoma | |||
| Mitral valve prolapse | Bronchogenic carcinoma squamous cell type | |||
| Hypertrophic subaortic stenosis | Empyema | |||
| Pleural malignant mesothelioma | ||||
| Thoracic actinomycosis |
Myocarditis and endocarditis have a low incidence rate as well as a wide variety of symptoms that can be associated with many other common ailments. Other than the high-risk factors for endocarditis, these two areas of infection are difficult to detect in the pre-hospital environment. Awareness of endocarditis and myocarditis is a tool for the pre-hospital clinician in that they have another form of differential diagnosis for these symptoms.
What the ambulance staff can contribute with regards to IVDUs with a differential diagnosis of endocarditis is that they are able to assess the surroundings. By being invited into the environment the patient lives in, ambulance staff are able to assess how the patient lives. Cleanliness of the needle the patient is using is important to document as the paramedic will be able to ask himself/herself are the needles left all over the floor for them to pick up bacteria from the carpet? Does the environment involve pets and the possibility of animal faeces around needles? Other indications that could be observed include: are there any of the patient's friends present that could come out with quotes such as: ‘It's the same sort of thing as our other friend had who ended up in hospital?’ These sorts of observations could help hospital staff assess the risk factor of the patient for endocarditis.
The amount of extra observations the paramedic can contribute towards myocarditis in children is probably less than endocarditis in IVDUs. Sudden cardiac deaths can only be managed by life-support protocols without the luxury of time to consider differential diagnosis. If a patient is not in this time-critical condition and shows signs of hypoperfusion, ambulance staff can document their suspicions. What could be contributed in this sort of scenario is that the differential diagnosis has been started earlier in the assessment process. If the hospital clinicians agree, the time taken to initiate tests for a diagnosis of myocarditis could be reduced.

