Renal calculi are common in the UK with an incidence of 2–3%, with 0.5% of the population experiencing an acute episode of renal colic every year (British Association of Urologic Surgeons, 2015). These episodes are responsible for over 12 000 hospital admissions yearly and incidence rates have been rising (British Association of Urologic Surgeons, 2015). Anecdotal evidence suggests that these patients are seen commonly in the pre-hospital environment; however, due to diagnostic uncertainty they are often conveyed to the emergency department.
This article seeks to evaluate the current evidence for diagnosing renal calculi in the pre-hospital environment and address the requirement for, and timing of, computerised tomography (CT) scanning in the renal colic patient. The case study discussed is an example of confident diagnosis resulting in appropriate management and avoidance of unnecessary hospital admission.
Case study
The case study describes a 48-year-old female who called 999 with symptoms descriptive of renal colic. The pain had started during the night, coming in waves and had worsened throughout the morning. The patient described her pain as sharp with associated nausea but no vomiting. Her previous medical history consisted of chronic obstructive pulmonary disease, previous mastectomy and a left-sided kidney stone 2 years earlier. Observations revealed a respiratory rate of 21 breaths per minute, heart rate of 98 bpm, blood pressure of 152/90 mmHg, oxygen saturations of 98% and a temperature of 36.8°C. On inspection, the patient's abdomen showed no signs of scars, peritoneal bruising or asymmetry. Auscultation revealed active bowel sounds; percussion, tympany throughout and palpation elicited mild tenderness over the site of pain and over the right renal angle.
The patient was asked to provide a urine sample, which showed microscopic haematuria with no other abnormalities and was graded using the STONE score (Schoenfeld et al, 2014), scoring 8—medium risk of uncomplicated renal calculi. These findings, combined with the history and patient assessment, led to a working diagnosis of renal calculi. The patient was managed with oral morphine, ibuprofen, supplied ongoing analgesia and a GP referral for ongoing investigations. In addition, she was advised that the course of kidney stones was normally self-limiting and given health and worsening advice. Reflection of this incident prompted further investigation into the subject matter.
Search strategy
A two-part question was used to form the basis of this literature search: i) can the STONE (Sex, Timing, Onset, Nausea and vomiting, Erythrocytes) score be used to diagnose renal calculi pre-hospital? ii) is CT required for renal colic? An extensive literature search was carried out on Medline, Cinahl, BNI and Embase, limiting results to English language trials, adult population trials and trials carried out in the last 10 years. The search yielded 536 results, each of which were browsed for relevance to the two-part question, duplicates removed and their references reviewed. Searches of the NHS evidence database (www.evidence.nhs.uk) and the Cochrane Database of Systematic Reviews (www.cochrane.org) were also completed. A data extraction sheet of results was compiled to allow easy comparison of study outcomes and authors' views.
The STONE score
Studies relating to the STONE score (Moore et al, 2014) were limited to four, two of which described the original study in different publications and two that validated the rule with varying results. The relatively recent concept of this rule may account for the limited evidence available to support its use. The original derivation and validation of the STONE score, as described by Moore (2014) and Bechis and Eisner (2014), when reviewed using the Clinical Appraisal Skills Programme (CASP) clinical prediction rule checklist (CASP, 2013), proves to be methodologically sound. It was blinded in all areas of the process, appropriate exclusions were made, and it had clearly defined patient groups and appropriate statistical methods. The study's resulting variables can each be validated through a wealth of existing literature, and the study had sufficiently high results to warrant reviewing practice.
| STONE score by factors and categories | Points |
|---|---|
| Sex | |
| Sex: | |
| Female | 0 |
| Male | 2 |
| Timing | |
| Duration of pain to presentation: | |
| >24 hours | 0 |
| 6–24 hours | 1 |
| <6 hours | 3 |
| Origin | |
| Race: | |
| Black | 0 |
| Non-black | 3 |
| Nausea | |
| Nausea and vomiting: | |
| None | 0 |
| Nausea alone | 1 |
| Vomiting alone | 2 |
| Erythrocytes | |
| Haematuria (on urine dipstick) | |
| Absent | 0 |
| Present | 3 |
| Total | |
| 0–5 = low risk, 6–9 = medium risk, 10–13 = high risk | |
The key variables of ‘Sex’, ‘Timing’, ‘Origin’ and ‘Nausea’ are well established in the evidence with little variance across citations. Haematuria on urine dip as a diagnostic sign is controversial, with sensitivity ranging from 85–90%. (Stewart and Joyce, 2008; Xavier and Maxwell, 2011). However, it is noted that 40% of flank pain and haematuria patients do not have kidney stones (Bultitude and Rees, 2012). As a result, most systematic reviews and guidelines on the topic suggest that haematuria alone cannot predict the presence or absence of renal calculi (National Institute for Health and Care Excellence (NICE), 2015). The STONE score accounts for this within its scoring system, ensuring that a patient with flank pain and isolated haematuria will receive a low score and thus prompt the search for alternative diagnoses.
Validation studies have received varying outcomes. The STONE score performed well in the study by Schoenfeld et al (2014); however, when applied by Wang et al (2015), it resulted in considerably poorer prediction rates. When analysing the cause of this, choice of cohort participants was key. The study by Wang et al (2015) derived its cohort from a previous study comparing the efficacy of ultrasonography and CT, implying that many of its participants are likely to have had other suspected, or known, diagnoses and may have had red-flag signs and symptoms present. Additionally, it included older patients and had a broader definition of dangerous alternative findings than the STONE score. The study by Schoenfeld et al (2015), however, unlike the original STONE study, excluded patients >50 years and those with red-flag signs. As a result, it received higher prediction percentages and lower dangerous alternative diagnoses than either of the other studies.
Neither the STONE score nor the subsequent validation studies addressed the impact of clinician gestalt or clinical decision-making on the reliability of the STONE score, thus having a possible negative impact on study outcomes. Daniels et al (2013) and Pierre and Nadel (2009) have conducted studies to assess the emergency provider's ability to predict renal calculi with positive results, suggesting that emergency department (ED) providers have a high rate of success at predicting the likelihood of renal calculi. These studies are small, do not specify the skill level of ED provider, and are limited in their methodology, thus limiting their applicability. However, anecdotal evidence and other established studies into clinician gestalt (Penaloza et al, 2013) support their findings.
Given the STONE score's already high validation results and low risk of dangerous alternative findings, it is possible to recommend that with the addition of clinician gestalt, thorough assessment and removal of patients known to be at risk, it could be used to make a confident, low-risk diagnosis of renal calculi in the urgent care environment.
CT scanning
If it is possible to diagnose renal calculi in the pre-hospital environment using the STONE score and clinician gestalt/decision-making as discussed above, is CT scanning required? And if so, when?
Of the 16 articles found to address CT requirement for renal colic, none were carried out in the pre-hospital environment, and as such may lack understanding of the demands placed on pre-hospital clinicians in relation to availability of imaging, length of time with the patient and pressure to avoid ED admission. The evidence was, however, unanimous in its research goal of reducing radiation doses by minimising CT scans, thus making most studies applicable to this topic.
The recent research appears divided as to whether initial CT scanning for patients aged 18–50 years is required (Zwank et al, 2013; Schoenfeld et al, 2014), with the primary concern relating to the likelihood of dangerous or acutely important alternative pathologies. Thirteen of the articles addressed the finding of alternative pathologies either as a primary or secondary outcome, with these figures found ranging from 0% (Schoenfeld et al, 2014; 2015) to 15% (Hoppe et al, 2006).
Studies that resulted in higher percentages of alternate pathologies were noted to include patients >50 years who are at higher risk of dangerous differential diagnoses, such as leaking abdominal aortic aneurism, malignancies and visceral ruptures. Conversely, they described infectious pathologies as a dangerous alternative finding (Cullen et al, 2008), when these could be identified by recognising the systemic signs of infection (Dellinger et al, 2013) or a urine dip positive for leukocytes. Studies that excluded patients >50 years, with known malignancy or with signs of infection, resulted in a range of dangerous pathologies found on CT between 0% (Schoenfeld et al, 2014; 2015) and 1.6% (Daniels et al, 2012). These figures are in line with the STONE score (Moore et al, 2014) outcomes, thus providing confidence that diagnosis without initial imaging should be possible.
While the diagnosis of renal calculi may be possible without imaging, the management of this condition without it is not supported in the evidence. The European Association of Urology (EAU) guidelines (Türk et al, 2015) and NICE (2015) clinical knowledge summary (CKS) both state that imaging to determine stone size and location is required to inform the requirement of urologic intervention. This recommendation can be assessed by looking at the likelihood of urologic intervention as a result of CT scanning, an outcome which is discussed in a number of studies.
Figures from these studies ranged from 2.4% (Schoenfeld et al, 2015) to 37% (Patatas et al, 2012) urologic interventions carried out as a result of CT scan. These studies ranged in size, outcome measures and location, and may be affected by differences in local guidelines for intervention, individual patient comorbidities, clinician belief in ‘watchful waiting’, or differing definitions of intervention. They do, however, as a combined weight of evidence, indicate that imaging of renal calculi does result in intervention.
Stone size and location have long been established as key factors in the likelihood of spontaneous passage, with some texts stating anywhere from >5 mm–> 8mm needing assisted removal (Türk et al, 2015). While the likelihood of spontaneous passage falls outside the remit of this literature review, it is clear that in the absence of a pre-hospital tool or procedure to assess stone size or location, referral for imaging must be made.
There is a paucity of evidence addressing the timing of this imaging. The EAU guidelines (Türk et al, 2015) and NICE (2015) CKS suggest that in a patient with adequately managed pain and no red flags, an urgent referral should be made for imaging within 7 days. This advice is based on expert opinion alone without explanation; however, it is possible that it is an acknowledgement of the current demands on today's health care and subsequent inability to action urgent referrals sooner than this time frame.
Three studies were found assessing the appropriateness of delayed CT scan for the renal colic patient; however, none were without significant limitations. The study by Schoenfeld et al (2014) states that delayed or no CT would be possible in young patients with renal colic. However, this study merely draws conclusions from a retrospective review of adverse findings on CT, and as such has not adequately addressed the positives and negatives of delayed CT scans. The study by Lindqvist et al (2006) is small, addressing only morbidity, intervention and revisits, but has strong methodology using randomisation and relevant inclusion criteria. The study comes to the conclusion that morbidity is no different for those CT scanned immediately, compared to those receiving delayed CT. The study by McAnulty et al (2013) bears the closest resemblance to this case study by evaluating a direct-access renal colic pathway for general practitioners. However, this study had poor results, indicating that this pathway did not reduce attendances at A&E, showed poor prediction rates from primary care and did not assess adverse outcomes from utilisation of the pathway. Patients referred through this pathway were not seen within a given timeframe, and as such prediction rates were variable for the GP cohort. However, this rate improved if patients were imaged within 24 hours. The poor results of this study can arguably be attributed to the authors' interpretation of the results, and structure of the study, rather than a fault in the concept of a direct-access renal colic pathway.
All three studies, despite limitations, indicate that delayed CT scanning for certain patients is appropriate and is not associated with higher morbidity or adverse findings. While the evidence alone is insufficient to make strong recommendations for delayed imaging, the lack of evidence to suggest harm in certain patients, along with the guidelines based on expert opinion, make it possible to recommend that patients <50 years, with adequately managed pain and no red flags, receive delayed imaging.
A flow chart to reflect the findings of this literature review (Figure 1) has been derived; it is hoped that this flow chart may form the basis for alternative care pathways in the pre-hospital environment in the future. This flow chart needs further testing before integration into practice, but sums up the current findings of this review.
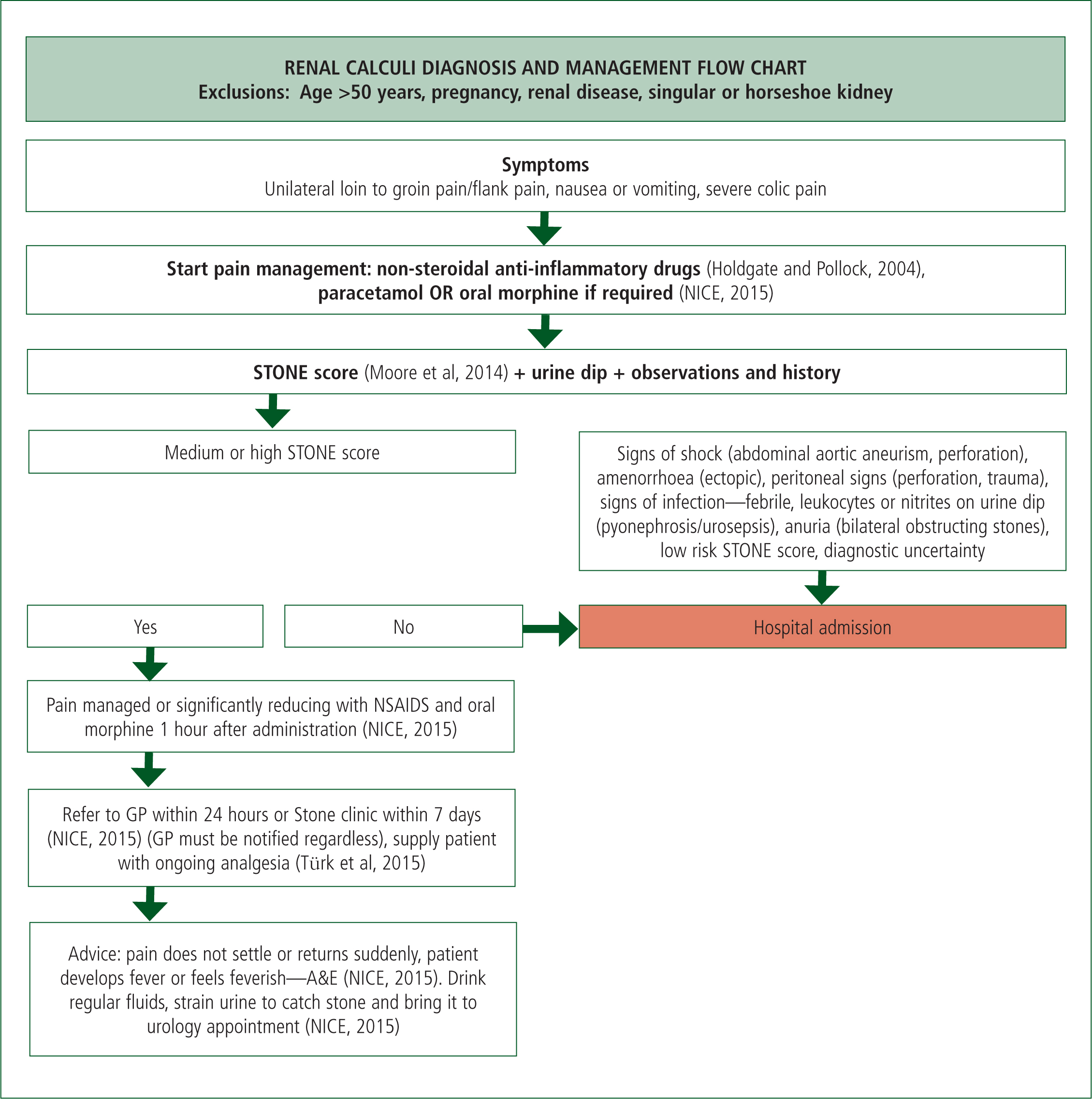
Conclusions
After addressing the evidence for the diagnosis of renal calculi and the subsequent imaging of this condition some recommendations can be made. The STONE score, when combined with clinical judgement and decision-making and applied to the right patient group, is an appropriate clinical decision tool to identify uncomplicated renal calculi. Once this working diagnosis is made, immediate CT imaging of this low-risk patient group is not required to confirm diagnosis; however, delayed CT scanning is required to form a management plan. The exact timing of this imaging has not been investigated in the literature so the current recommendations from the EAU guidelines should be followed until further studies are completed. By making these changes to practice, further unplanned hospital admissions might be avoided, thus making positive changes to the NHS.

