Ischaemic heart disease (IHD) is the leading cause of death worldwide with 12.8% of all deaths attributed to this (World Health Organization (WHO), 2011). A number of IHD patients may also develop an acute coronary syndrome (ACS). Ambulance clinicians commonly encounter patients suffering ACS, this being unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI).
Differentiating between the three can be challenging, however with good history from the patient, including history of the complaint along with established cardiac risk factors, familial history and a good understanding of electrocardiograms (ECGs), differentiating between STEMI and Non ST elevation acute coronary syndromes (NSTEACS) can be clinically suspected and achieved with blood biomarker assays.However, all three types of ACS would warrant the same drug therapy from paramedics and currently only STEMI patients attend heart attack centres (HACs) as standard practice where the patients may undergo primary percutaneous coronary intervention (pPCI). These patients undergo an assessment by a cardiologist and angiography commonly followed by re-vascularisation and stent insertion if required to open up the affected coronary artery/s. NSTEACS patients could be seen to be pre-STEMI as their stenosis may be critical and may develop into a STEMI, therefore benefiting from early expert opinion to optimise future heart function and if required revascularisation.
STEMI vs NSTEACS
This is not to say that all NSTEACS patients would require this aggressive intervention but the intermediate to high-risk patients ultimately may. The bypass of local emergency departments (ED) and going direct to a HAC may decrease mortality and morbidity. Various studies demonstrate between 6–12% of patients presenting with NSTEACS do not have significant coronary artery disease (CAD) (Papanicolaou et al, 1986; Pecora et al, 1988; Diver et al, 1994; Roe et al, 2000; Patel et al, 2006). However, this highlights that a significant number, possibly up to 94% do have CAD and may benefit from angiography and subsequent revascularisation. Those that do not require such invasive procedures immediately will only have been taken potentially further from their local ED.
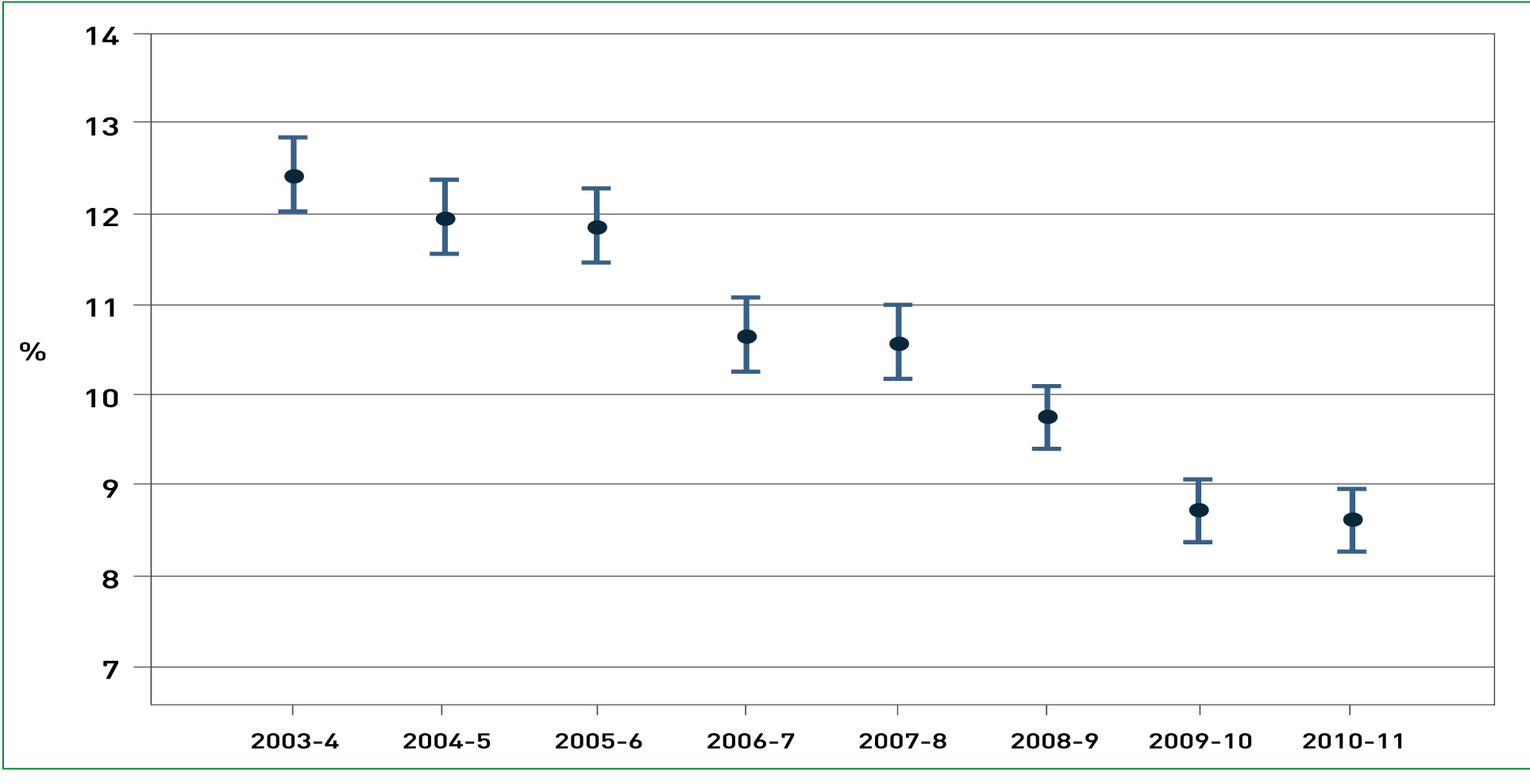
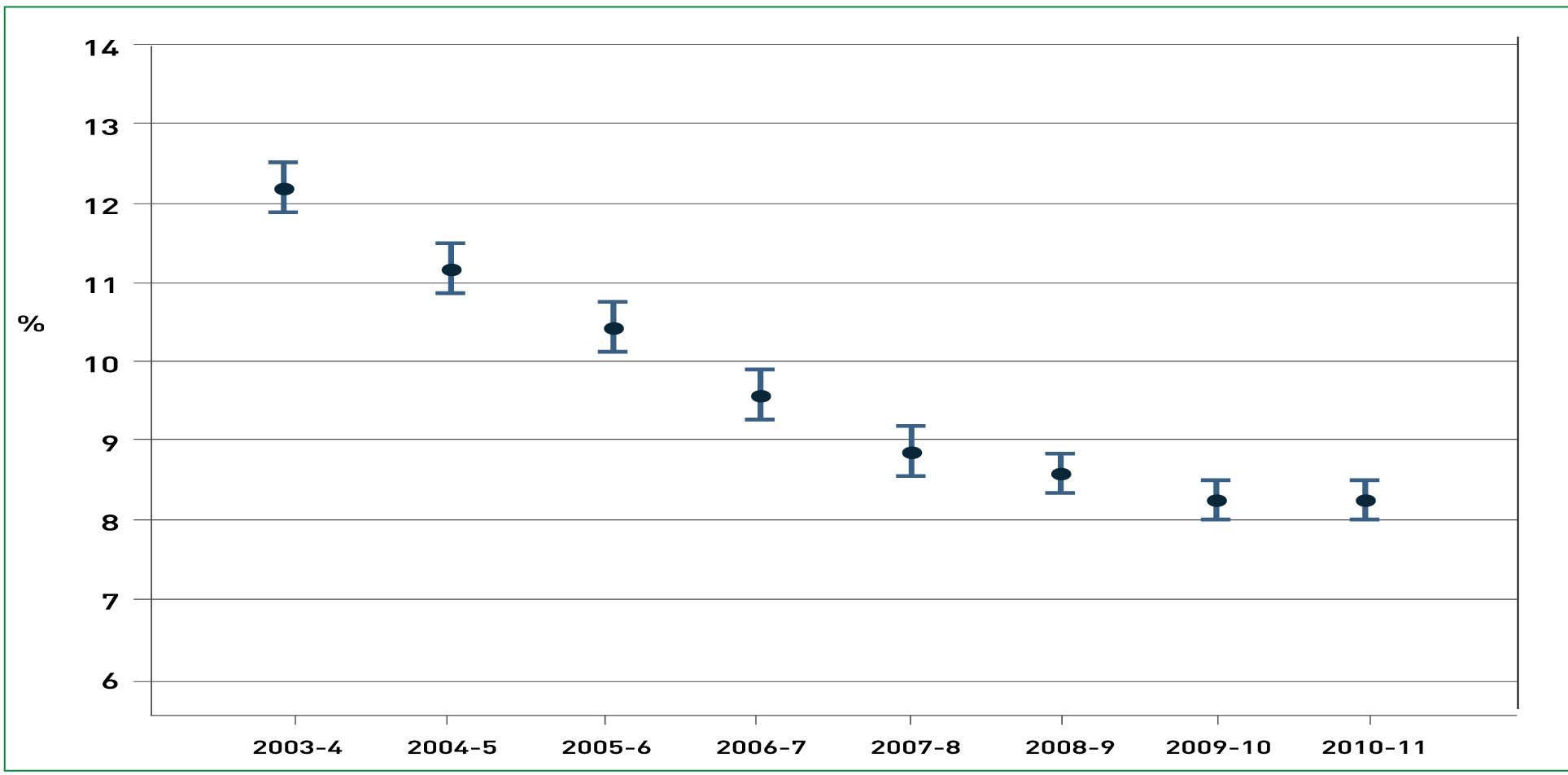
The mortality at six months post event in NSTEACS compared to STEMI is significantly higher at 8.9% compared to 6.8% (Savonitto et al, 1999). In another paper based in France, the one-year mortality of STEMI compared to NSTEACS was 9.0% and 11.6% respectively, thus highlighting that both types of MI may warrant urgent intervention (Montalescot et al, 2007). According to the MINAP report 2010/2011 approximately 60% of the heart attack admissions to hospitals in England and Wales were NSTEACS and the remaining 40% being STEMIs, it is recognised that frequently NSTEACS are not recorded accurately and the true ratio of NSTEACS to STEMI is 2:1 (MINAP, 2011). Nevertheless it is the STEMI patients that receive the early invasive procedures despite the fact that the 30-day mortality for each MI is approximately the same (MINAP, 2011) (Figure 1 and 2).
Risk stratification—TIMI risk score
When obtaining patient history it is also important to identify any risk factors that could make the potential diagnosis of ACS more likely and strengthen differential diagnosis. EDs often use a TIMI risk score for NSTEACS that incorporates many of the risk factors that ambulance clinicians should be obtaining in these potentially high-risk patients. The TIMI risk score is a statistically and clinically significant prognostic tool developed and tested to determine the outcome of death and ischaemic events (Antman et al, 2000). The patient obtains one point for every positive finding on the TIMI risk score; if not present or true then they gain zero points (Box 1). The higher the TIMI score the increased risk of mortality, MI or severe recurrent ischaemia requiring urgent revascularisation (Box 2).
| Low Risk | 0/1–4.7% |
| 2–8.3% | |
| Intermediate risk | 3–13.2% |
| 4–19.9% | |
| High Risk | 5–26.2% |
| 6/7–40.9% |
GRACE risk score
Global Registry of Acute Cardiac Events (GRACE) is a large multinational prospective observational study in which data is obtained from patients with ACS regarding their clinical status, interventions performed and follow up in an attempt to identify practice that is beneficial and ultimately to enhance patient care in this group of patients (GRACE Investigators, 2001). GRACE was launched in 1999 and currently as of the beginning of 2012, thirty countries are involved, 247 hospitals and 102 341 patients have been recruited (GRACE, 2012). There are two parts to the GRACE score, one performed in the acute setting at admission to hospital (Figure 3) and one performed at discharge from hospital (Figure 4).
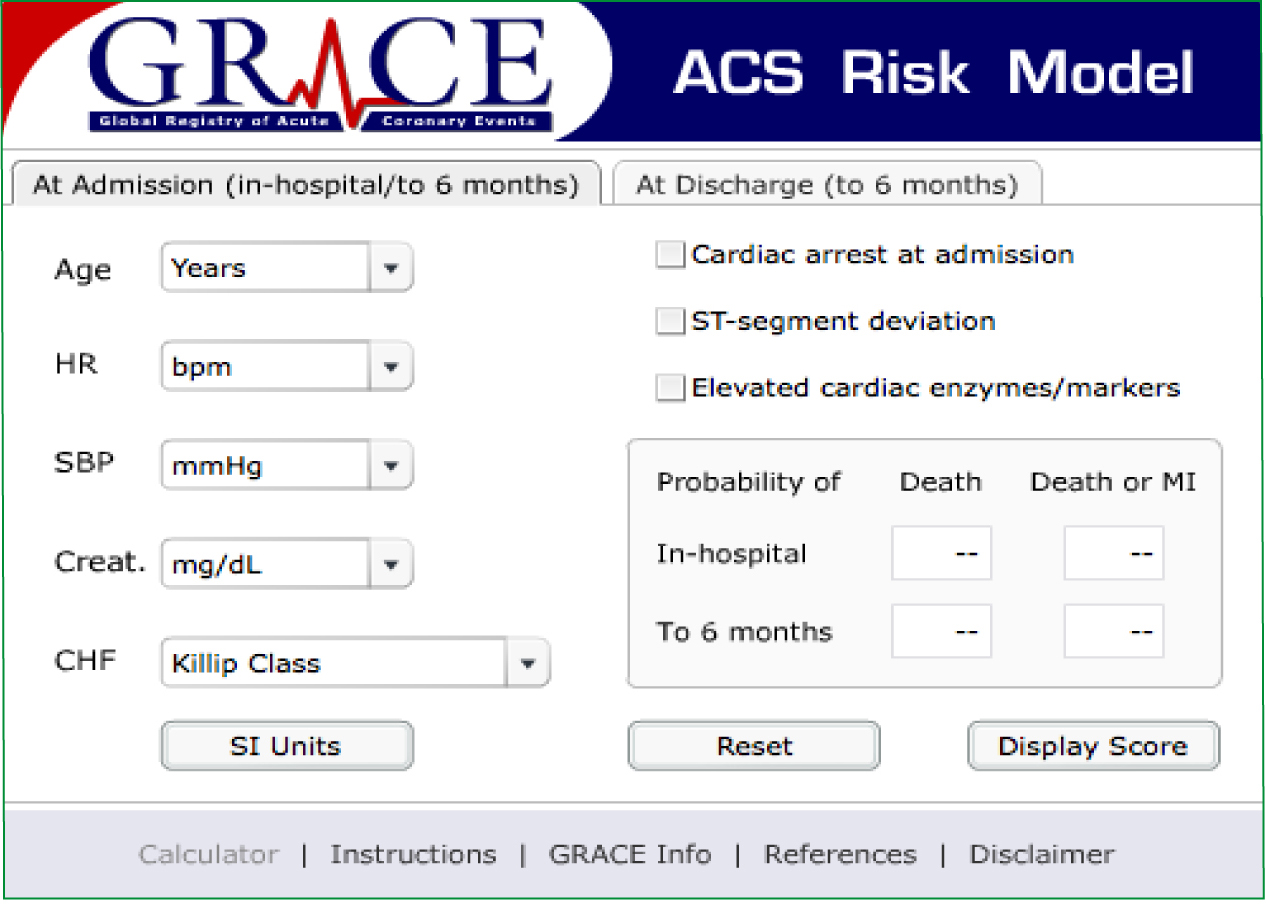
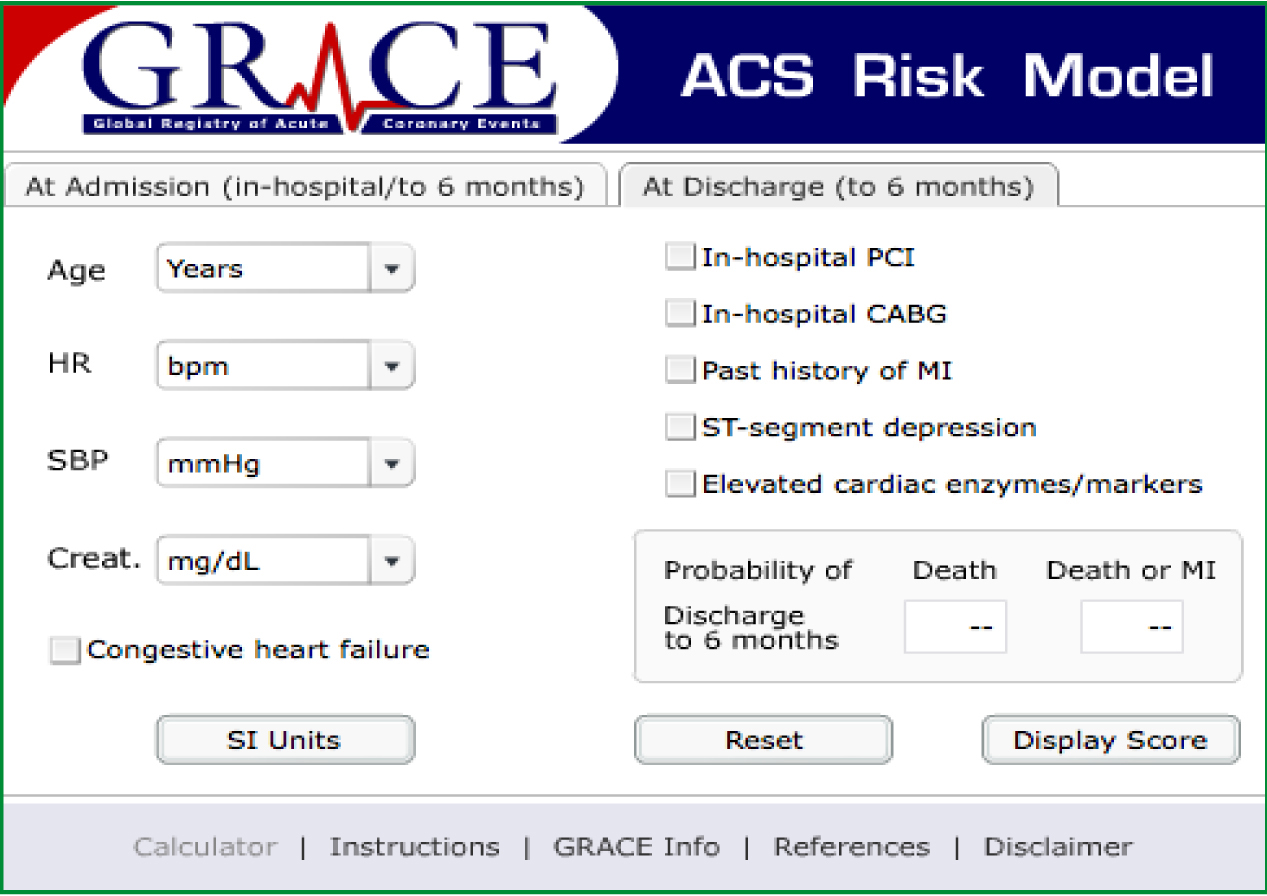
Both risk stratification tools are proven to be accurate at predicting mortality at 30 days, however some EDs use TIMI and others GRACE. This is as they are both recognised and TIMI is believed to be easier to use than GRACE in the ED (Lyon et al, 2007).
Ambulance clinicians do not routinely carry out blood tests in the field meaning the cardiac markers and creatinine of each risk scoring cannot be performed. In addition, it may never be required as different cardiac markers peak elevation at different times and would not rule out an ACS. Cardiac biomarkers complement good history, physical assessment and the ECG to affirm the diagnosis of ACS, however they must be used in conjunction with these, as they can also be elevated from other medical conditions unrelated to ACS. Furthermore, both risk stratification tools have been developed and tested and therefore validated in the in-hospital environment and none have been validated in the pre-hospital environment. That is not to say that some elements, if not the majority of them could be extrapolated to the pre-hospital field and used with ease, however they have not been validated so would not be considered an accurate way to risk stratify. Therefore a modifed GRACE or modified TIMI is a potential future project that may prove beneficial in the pre-hospital field for use by the paramedic profession once it has undergone testing and validation.
However, the other elements of the risk score can be used by ambulance clinicians and a threshold put upon low/medium/high risk patients that should be taken to HACs. A large multicentre clinical trial found statistically significant results that the young, female, black population who do not smoke, are not diabetics or do not suffer with hyperlipidemia are less likely to develop CAD (Patel et al, 2006). The TIMI risk score incorporates all of these factors excluding gender and ethnicity. It is well known that the South Asian population commonly have increased risk of cardiovascular disease when compared to their Caucasian counterparts. The main reason being the high proportion of the South Asian population who have non-insulin dependent diabetes mellitus and increased insulin resistance alongside their lipid profile consisting of low HDL and high levels of triglycerides (McKeigue et al, 2004; Ramaraj and Chellappa, 2008). Chest pain has been reported sometimes up to eight weeks prior to the coronary event, which could be considered to be a prodromal symptom that is very common in MI patients (Stowers and Short, 1970). Therefore, these factors may warrant inclusion into a risk stratification tool in the future.
There are a number of robust studies including meta-analyses and randomised controlled trials (RCTs) evaluating early invasive therapies including angiography and potential for PCI and non-invasive pharmacological management of NSTEACS. The main risk of performing the invasive procedures is the risk of bleeding and procedure related MI. However, these risks are counteracted with the additional benefits of decreased risk of death, non-fatal MI or re-hospitalisation in the medium to high-risk patients (Wallentin et al, 1999; Cannon et al, 2001; Fox et al, 2002; Hoenig et al, 2010).
Over the last eight years the use of angiography for NSTEACS patients has increased dramatically, in 2003/2004 it was 35.1% and in 2010/2011 it was 71% (MINAP, 2011). Consequently, if angiography has to be performed within 96 hours and 71% of patients will receive it then minimising ED assessment and tests with a referral and discharge seems logical considering they will receive the same tests plus a higher level of cardiology speciality care in a HAC.
The balance between risks of invasive procedure against conservative pharmacological intervention needs to be carefully managed by the clinician (NICE, 2010). It is not the decision of the paramedic as to which modality of treatment is best for the patient. However, if paramedics can recognise the intermediate to high-risk patients and convey them to an appropriate facility, the cardiology physician using sophisticated tests can assess risk and benefits making the pre-hospital management remarkably simple.
Furthermore, age should not be a factor in deciding whether the patient should undergo this procedure. Devlin et al (2008) highlight that patients with high risk NSTEACS of 70 years and above still benefit at 6 months from revascularisation similarly to their younger counterparts with no increase in stroke risk. Hence, these patients should still be managed in the same manner and the cardiologist at the centre may subsequently assess the risk/benefits of the procedure if necessary.
The NICE guidance for NSTEACS suggest that angiography with the option of PCI should be offered within 96 hours of hospital admission if they have a predicted six month mortality above 3.0% using a risk stratification tool such as GRACE (Table 1, 2), this being the intermediate and high-risk patients (NICE, 2010). They also state that angiography should be performed as soon as possible for clinically unstable or high-risk ischaemic patients. The EDs may be using this guidance and once it is ascertained that the patient is intermediate to high-risk they may be taken to the cardiac catheterisation suite or an inter-hospital transfer would be booked to take the patient to a HAC. If ambulance clinicians can recognise these high-risk patients and are able to convey direct to a HAC, this will ultimately optimise compliance with the guideline to provide high quality patient care. It is evidenced that the longer angiography is left in NSTEACS patients the higher the risk of recurrent in-hospital ischaemia, re-infarction, and heart failure (Swanson et al, 2009). It is evident that even late angiography at the mean time of 46 hours or > 72 hours or the agreed NICE guideline of <96 hours is preferable to not receiving it at all, nevertheless the earlier is arguably better in this subset of patients (Swanson et al, 2009).
| Risk category | GRACE risk score | Probability of death in hospital |
|---|---|---|
| Low | 1–108 | <1% |
| Intermediate | 109–140 | 1–3% |
| High | 141–372 | 1–3% |
| Risk category | Grace risk score | Probability of death in hospital |
|---|---|---|
| Low | 1–88 | <3% |
| Intermediate | 89–118 | 3–8% |
| High | 119–263 | > 8% |
| Observations |
|
| Past medical history | Arthritis |
| Medicines | Ranitidine, Ibuprofen, Paracetamol |
The case
A 75-year-old Asian male rang 999 as he was complaining of sudden onset, sharp, central chest pain, which began on mild exertion. The pain radiated slightly through to his back and the patient reported a pain score of 8/10. The patient was nauseous, vomited once and complained of shortness of breath, although did not appear notably dyspnoeic. The patient had pallor, was clammy and at times was displaying Levine's sign. The patient stated that he had chest pain for a few days intermittently and had visited his general practitioner (GP) who advised the patient to take analgesia. The patient followed the advice given. The patient did not have a history of CAD; he was not a diabetic or hypertensive patient and did not smoke. He was unsure of any family history of CAD.
The patient's ECG showed right bundle branch block (RBBB), ST segment depression in II, a VF, V2, V3, V4, V5 with inverted T-waves also in III and V1 (Figure 5). The tall R-wave in V1 and ST depression could have indicated a posterior infarct; hence a posterior view was undertaken. This view did not show any ST-segment elevation so posterior infarction was unlikely. ST-segment elevation was absent in the inferior view hence the need to acquire a right sided ECG was not indicated. Patients with insignificant CAD do not generally have ST-segment changes upon presentation at the receiving hospital (Patel et al, 2006). Hence, given the degree of ST deviation it was likely he was suffering significant coronary artery stenosis. Based upon the TIMI risk score the patient would have scored three out of seven based upon his age, ST depression and anginal chest pain in the last 24 hours. Hence, he had a 13.2% risk of a serious coronary event. The patient also had RBBB, regardless of this being old or new, the intraventricular conduction delay is clinically significant of a more extensive MI and carries a higher prognosis of death and mortality at 30 days (Wong et al, 2006). Some authors only state this is true of the STEMI patient with RBBB whereas others are less specific and state ACS with RBBB carries the same risk (Horton and Brady, 2009). Kleeman et al (2008) state there is no relationship between NSTEACS and RBBB leading to increased mortality risk, hence this is a contentious topic that should be considered when reviewing risk of morbidity.

Based on the clinical presentation, clinical history and ECG, the patient was assumed to be having a NSTEACS and was given 300 mg aspirin orally, 400 mcg GTN sub lingually and 4 mg morphine intravenously. The patient was no longer feeling nauseous and had not continued to vomit. The patient's pain reduced with GTN to 5/10 and morphine was given soon after the pain score was obtained in an attempt to further reduce the patient's pain.
Given the clinical picture, the patient was conveyed direct to a HAC rather than an ED as it was deemed clinically significant by the ambulance clinicians on scene to provide the best quality patient care. Angiography revealed severe mid vessel stenosis in the left anterior descending (LAD) artery and a severe mid vessel lesion in his right coronary artery (RCA). Both underwent pPCI with a drug eluting stent with a good end result.
The evidence
There are a number of RCTs, commonly large multicentre prospective trials surrounding early invasive angiography with revascularisation (if required) compared to a conservative (selective invasive) approach to NSTEACS management. These RCTs also form a large part in a series of meta-analyses in this contentious field. It is not the aim of this paper to further analyse the papers extensively; however highlighting the key papers and issues is worthwhile to gain a full understanding of the current and potential future management of this group of patients.
The main trials upon which the current guidelines are based and the meta-analyses focus upon are the FRISC II (Wallentin et al, 1999), TRUCS (Michalis et al, 2000), TACTICS-TIMI 18 (Cannon et al, 2001), RITA-3 (Fox et al, 2002), VINO (Spacek et al, 2002), ISAR-COOL (Neumann et al, 2003), ICTUS (De Winter et al, 2005), OPTIMA (Riezebos et al, 2009), and TIMACS (Mehta et al, 2009) (Table 3). These are not exhaustive as there are other robust trials however these are the majority of the trials. Each trial has its own limitations and all used slightly different parameters of investigation such as the use of clopidogrel in 300 mg or 600 mg dose or the use of glycoprotein IIb/IIIa antagonists, the differentiation between diagnoses of MI based upon ECG, chest pain or solely an elevated CK-MB. Furthermore, in several of the trials many of the patients in the conservative/delayed invasive therapy arms underwent angiography and revascularisation only slightly after the early invasive arm. Other trials that consider the intervention as early, some may label as delayed, so a degree of crossover exists, sometimes as high as 56% (Hamm et al, 2011). Some trials used alternative drugs as more advanced drug therapies and practices were developed. It is for that reason that a few of the earlier RCTs such as VANQWISH and TIMI IIIB were not included in many meta-analyses as they were conducted before the routine use of triple antiplatelet therapy and use of stents (TIMI IIIB Investigators, 1994; Boden et al, 1998). All trials had a fair distribution of participants with regard to baseline characteristics between either treatment arm and all used the same general criteria of entry.
| FRISC II | Fragmin and fast revascularisation during instability in coronary artery disease. |
| RITA-3 | Randomised intervention trial of unstable angina. |
| TACTICS-TIMI 18 | Treat angina with aggrastat and determine the cost of therapy with an invasive or conservative strategy—thrombolysis in myocardial infarction. |
| VINO | Value of first day coronary angiography/angioplasty in evolving non-ST segment elevation myocardial infraction. |
| ISAR-COOL | Intracoronary stenting with antithrombotic regimen cooling off. |
| OPTIMA | optimal timing of PCI in unstable angina. |
| TRUCS | treatment of refractory unstable angina in geographically isolated areas without cardiac surgery. Invasive versus conservative strategy. |
| ICTUS | Invasive versus conservative treatment in unstable coronary syndromes. |
| TIMACS | Timing of intervention in acute coronary syndrome. |
| VANQWISH | Veterans affairs non Q-wave infarction strategies in hospital. |
| TIMI IIIB | Thrombolysis in myocardial ischemia. |
The FRISC II, TACTICS TIMI 18, VINO, TRUCS and ISAR-COOL trial demonstrated a decreased incidence of major cardiac events, this commonly being MI, death or recurrent ischaemic events when an early invasive strategy was compared to a selectively invasive or conservative strategy (Wallentin et al, 1999; Michalis et al, 2000; Cannon et al, 2001; Spacek et al, 2002; Neumann et al, 2003). These results can also be seen to be beneficial at 30 days and at other follow up points this being six months or yearly, or even at five yearly with the FRISC II trial (Lagerqvist et al, 2006). This can also be extended with the addition of the TIMACS trial, which did not show a beneft with an early invasive strategy initially but showed a reduction in secondary death, MI or refractory ischaemia (Mehta et al, 2009). Furthermore, a signifcant reduction in death or MI was demonstrated in this trial at 6 months follow up in the early invasive arm in patients who were high risk, namely a GRACE score of > 140 (14.1 vs 21.6%, P=0.005) (Mehta et al, 2009). The RITA-3 trial showed benefts such as a large reduction in anginal symptoms in the early intervention group with no increased risk of MI or death, furthermore the health related quality of life is said to be improved in the early intervention group after one year post intervention (Fox et al, 2002; Kim et al, 2005). Performing early invasive therapy reduces mortality, but three of the seven RCTs performed the early invasive therapy after 24 hours which revealed a further reduction in mortality, highlighting that possibly pPCI may be delayed for at least 24 hours, but not longer than 48 hours (Bavry et al, 2006). The major fndings of the meta-analysis of the contemporary RCTs was non-fatal MI was reduced by 17% and the number needed to treat (NNT) was 66 patients to prevent one fatal event. Furthermore, recurrent unstable angina requiring rehospitalisation was reduced by 31%, to prevent recurrent unstable angina the NNT is 11 (Bavry et al, 2006).
‘The question that needs further research is;‘what is the best time to take these high-risk NSTEACS patients for PCI?’
The Cochrane Collaboration basing their decision upon a number of trials concluded that an early invasive strategy reduces rehospitalisation, refractory angina and MI. However, the early invasive strategy increases the risk of periprocedural MI and biomarker leaks so it would be best performed in the high-risk group of patients (Hoenig et al, 2010). In a meta-analysis by Choudhry et al (2005) they comment that patients with ST segment depression have a greater beneft from an invasive strategy. They also comment that having an elevated cardiac biomarker at admission did not show any further improvement by an invasive strategy, which supports the theory that pre-hospital cardiac biomarker testing is not necessary (Choudhry et al, 2005). Nonetheless, in other trials such as the OPTIMA trial immediate PCI compared to the apparently delayed PCI of 24–48 hours increased the rate of MI, commonly periprocedural, which led the authors to conclude that PCI should be delayed for at least 24 hours (Riezebos et al, 2009). Furthermore, the ICTUS trial also established that an early invasive strategy (within 24–48 hours) was not superior to a selective invasive strategy with regard to death or MI however rehospitalisation of the early invasive group was reduced (De Winter et al, 2005). It should be noted that the parameters set in the ICTUS trial regarding diagnosis of MI were very sensitive, this meaning only an elevated CK-MB of more than one above the upper limit of normal was considered an MI. Furthermore, blood was drawn every 6 hours post procedure for 24 hours, if other trials had used this parameter they may have found an increased incidence of MI also (Bavry et al, 2006). Conversely, the ISAR-COOL trial demonstrated that delayed coronary intervention for antithrombotic therapy of a medium time of 86 hours compared to the medium time of the early invasive arm of 2.4 hours did not improve the outcome, hence they conclude that the shorter the period before catheterisation the lower the incidence of adverse events (Neumann et al, 2003). The main question is not ‘should high-risk NSTEACS patients receive PCI?’ as it is well documented that they need the intervention and even very delayed intervention (>72 hours) is better with regard to reducing adverse cardiovascular events such as stroke, MI or death than receiving no PCI at all (Swanson et al, 2009). The question that needs further research is; ‘what is the best time to take these high-risk NSTEACS patients for PCI?’. The guidelines differ somewhat between countries despite all being based upon the same clinical trials, which demonstrates the complexity of this intervention. The European Society of Cardiology (ESC) have four tiers of strategy, this being invasive (< 72 hours), urgent invasive (< 120 minutes), early invasive (< 24 hours) or primarily conservative (Hamm et al, 2011). The NICE guidelines suggest that the invasive strategy should be performed within 96 hours, so somewhat longer than the ESC guidelines and arguably this is not ‘early invasive’ if performed close to the 96 hour period (NICE, 2010). The American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) are less quantitative in their recommendations, stating that patients at high-risk will undergo angiography between 4–24 hours or if deemed to be immediate they will receive the revascularisation within minutes to hours (Wright et al, 2011).
If a patient is believed to be suffering from NSTEACS based upon patient history including cardiac sounding chest pain, risk factors for CAD and an ECG showing ST segment deviation, then the next step is the need for a pre-hospital validated risk stratification tool to determine if the patient is low, intermediate or high risk. The evidence suggests that low risk beneft from conservative medical management and the intermediate to high-risk benefit from angiography and potentially revascularisation (Hamm et al, 2011). If the pre-hospital clinician used a tool and stratified the patient as intermediate to high-risk then the advanced assessment provided by a cardiologist using ultrasound, blood biomarkers, validated risk stratification tools, angiography facilities and advanced drug therapies, must be better for patient care than a clinician not specialised in cardiology.
Given the ERC guidelines of < 24 hours for early invasive strategies for high-risk and < 72 hours for less acute risk or conservative strategies for low risk patients, the NICE guidelines is 96 hours for any patient could be viewed to be too long (NICE, 2010; Hamm et al, 2011). The earlier these patients receive specialist input in a specialist centre the better their outcome. Pre-hospital clinicians can play a significant role in reducing the workload of regional EDs, and thus increasing the work of HACs with the benefit of decreasing the morbidity and mortality of these patients.
