Serotonin toxicity is an increasingly common and potentially life threatening medical condition caused by an excess of the neurotransmitter serotonin. The condition can occur as a consequence of a drug overdose, drug interaction or as the result of an adverse drug reaction (Boyer and Shannon, 2005).
Serotonin toxicity is characterised by a triad of neuroexcitatory signs—namely, mental status changes, autonomic hyperactivity and neuromuscular abnormalities. This triad occurs due to an increased level of intrasynaptic serotonin which in turn causes excessive stimulation of serotonin receptors (Dunkley et al, 2003).
Statistics on serotonin toxicity cases within the UK are sparse but Bronstein et al (2010) found, on analysis of poison centre data from the USA, that there were 48 204 exposures to selective serotonin re-uptake inhibitors (SSRIs) (one of the most common medications associated with serotonin toxicity) in which 1 382 patients developed serious symptoms and 97 died.
The mortality rate for severe serotonin toxicity is estimated to range from 2–12% (Frank, 2008). Research has also shown 14–16% of patients who overdose on SSRIs show some clinical features of serotonin toxicity (Isbister et al, 2004). The condition has been observed in all age groups with some authors suggesting that the elderly are at an increased risk due to polypharmacy and inadvertent prescribing of several different serotonergic medications (Poeschla et al, 2011).
Serotonin toxicity is not a common presenting condition pre-hospital practitioners will face but one that needs prompt identification and treatment to prevent further deterioration. This article will explore the importance of early recognition and management of those patients presenting with clinical features indicative of serotonin toxicity.
Background
The condition was first described in medical literature by Oates and Sjoerdsma (1960). Oates found that patients developed certain neuroexcitatory features after receiving the amin acid tryptophan while taking a monoamine oxidase inhibitor (MAOI). Despite this early published literature, it has not been until more recent years that the condition has become far more prevalent (Isbister et al, 2007).
The rise of serotonin toxicity cases in recent years is in part due to an increase in the development and prescribing of serotonergic medications, mainly in the treatment of depression. In 2010, 22.7 million more items were dispensed in the community in England for antidepressant medications when compared with the same period for 1999 (Department of Health (DH), 2001; Information Centre, 2011).
This rise over time goes some way to explaining an increase in cases of serotonin toxicity being diagnosed in hospital. Another cause of an increase in cases is a rise in medical literature on the subject. A simple Medline and CINHAL search for the term ‘serotonin toxicity’ from 1960–2000 yields 314 results, whereas the same search for only the last 12 years from 2000–2012 yields 453 results suggesting an increase in interest and research on the subject, therefore making clinicians more aware of the condition as a possible diagnosis.
Despite this increase in literature the condition is still not widely recognised by Health Care Professionals (HCP). The current JRCALC (2006) guidelines make no reference to the condition and a survey of general practitioners in England found that 85% were unaware of serotonin toxicity as a possible diagnosis (Mackay et al, 1999).
The difficulty in diagnosis may be in part due to serotonin toxicity’s wide array of presenting clinical features, many of which could be attributed to other more common conditions, but also due to there being no laboratory test to accurately diagnose the condition. Diagnosis is based primarily on the patients’ history and presenting clinical features, which may range from mild and barely noticeable to severe and life threatening (Ng et al, 2008).
Pathophysiology and pharmacology
Serotonin, which is chemically known as 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter synthesized from the amino acid tryptophan. The chemical 5-HT is mostly found in the gastrointestinal tract (GIT) which is home to 90% of the bodies’ total 5-HT with the remainder found in the presynaptic neurons of the central nervous system (CNS) and to a lesser degree in blood platelets where it promotes platelet aggregation. The chemical 5-HT has many wide ranging effects on the body both centrally and peripherall including control of appetite, cardiovascular function, muscle contraction, sleep, memory, learning, temperature regulation, mood and behaviour, making 5-HT vital in many bodily functions (Berger et al, 2009).
There are several different groups of drugs that by using different pharmacological mechanisms cause an increase in the amount of 5-HT within the synapse causing the clinical features seen in serotonin toxicity. These mechanisms include monoamine oxidase inhibitors (MAOIs) which prevent serotonin metabolism, SSRIs which inhibit serotonin re-uptake into the presynaptic neuron (Figure 1), serotonin releasing agents (SRA) which induce serotonin release and finally increasing serotonin precursors such as tryptophan will increase serotonin levels within the synapse.
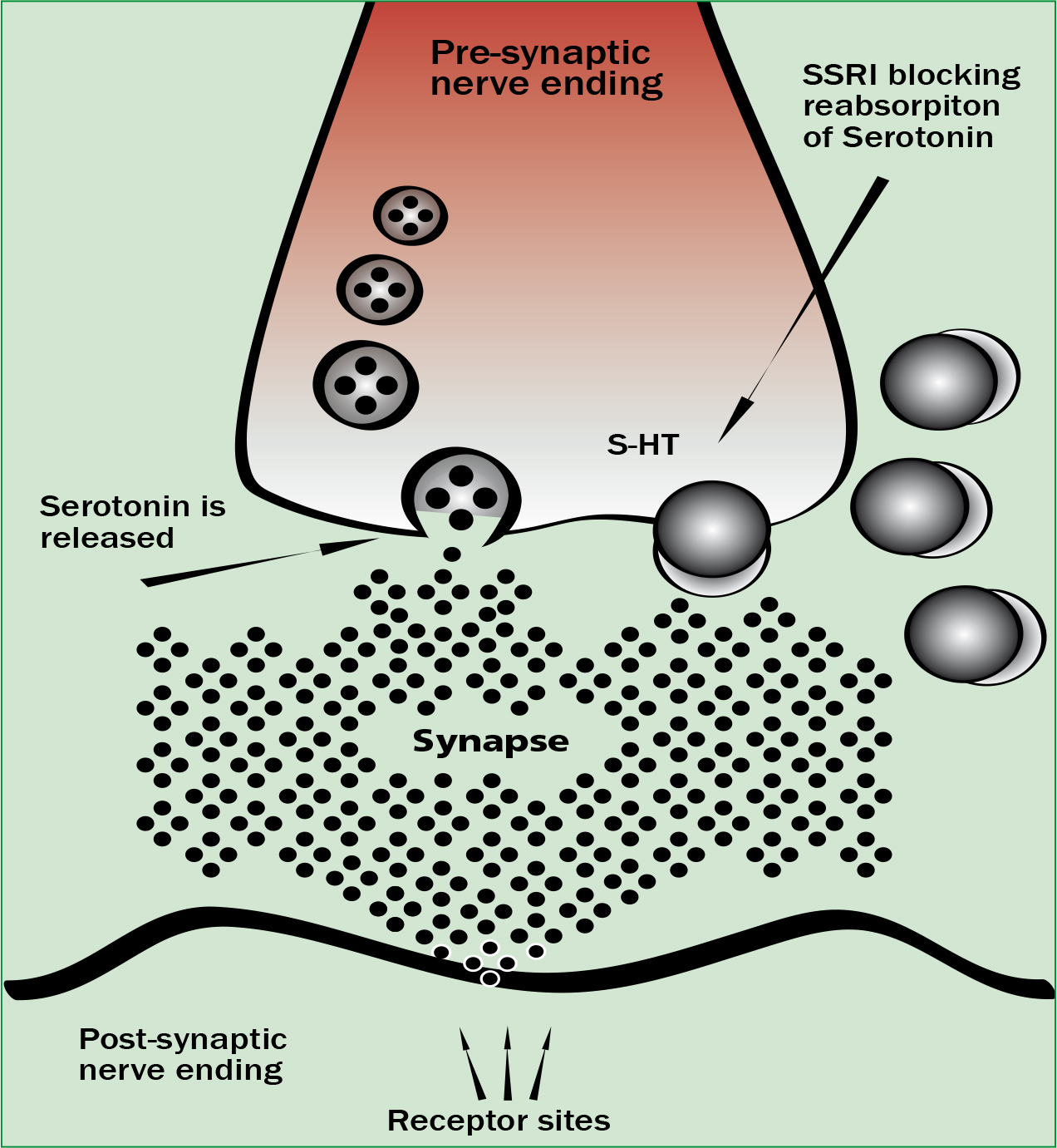
When taken individually in a normal prescribed dose serotonergic medications are unlikely to cause any clinical features of serotonin toxicity. Toxicity has been associated with taking single serotonergic agents such as SSRIs although rarely do severe or fatal cases result from taking just one agent (Frank, 2008; Boyer, 2012). Severe toxicity normally occurs when combining two serotonergic drugs with different pharmacological mechanisms. This can cause an increase in intrasynaptic serotonin to a level where severe features develop. There are only two groups of serotonergic agents known to cause severe and fatal toxicity. The first group are patients having taken a MAOI in combination with a SSRI and the second group is those having taken a SRA such as ecstasy combined with a MAOI (Gillman, 2005). Case studies have shown that SSRIs alone do not cause severe serotonin toxicity or a rise in temperature beyond 38.5 °C (Gillman, 2005).
Serotonin toxicity is not a condition that will occur naturally only as the result of taking one or more serotonergic agents. Therefore gaining a full history of all drugs taken is paramount in diagnosis of the condition. Table 1 shows a list of serotonergic drugs that have been associated with serotonin toxicity and may make the pre-hospital clinician consider it as a possible diagnosis. It is also important to remember that non-prescribed and illicit drugs can also cause the condition. Therefore the patient should be questioned about all drugs that they have been taking and not limited solely to physician prescribed medications.
| Selective serotonin reuptake inhibitors | Sertraline, fluoxetine, citalopram, paroxetine, fluvoxamine, escitalopram |
| Monoamine oxidase inhibitors | Phenelzine, tranylcypromine, moclobemide |
| Non-Selective monoamine reuptake inhibitors | Imipramine, clomipramine, amitriptyline, nortriptyline, doxepin, dothiepin |
| Selective serotonin (5HT1) agonists | Sumatriptan, naratriptan, zolmitriptan |
| Other serotonergic agents | Linezolid, pentazocine, dextromethorphan, sibutramine, fenfluramine, pethidine, fentanyl, tramadol, lithium, selegiline |
Clinical presentation
As previously discussed in this article, the patient’s history is paramount in diagnosis of serotonin toxicity. Clinical features start approximately 6 hours after an increase in serotonergic medications and onset is most often rapid (Hall and Buckley, 2003; Quinn and Stern, 2009). Clinical features of serotonin toxicity are highly variable but distinction is often made between mild, moderate and severe presentations.
The most common clinical features to observe in the diagnosis of serotonin toxicity are mental status changes, restlessness, myoclonus, hyperreflexia, diaphoresis and shivering (Sternbach, 1991). Table 2 summarises the main neuromuscular, autonomic and mental state changes seen with toxicity at different levels of severity. Being able to categorise a patient’s level of toxicity may be beneficial in deciding the patients treatment needs. It is important to remember that the condition can progress over several hours and a patient initially presenting with mild symptoms can rapidly deteriorate into severe toxicity (Isbister, 2007; Boyer, 2012). As a result of this, it is paramount that clinicians are prepared to aggressively manage any such deterioration. Walsh (2010) advocates the use of the mnemonic CAN (Box 1) when remembering signs and symptoms of serotonin toxicity.
| Autonomic signs | Neurological signs | Mental status changes | |
|---|---|---|---|
| Mild | Tachycardia |
Intermittent tremor |
Anxiety |
| Moderate | Diarrhoea |
Ocular clonus |
Easily startled |
| Severe | Temperature > 41 °C | Ocular clonus |
Coma Delirium |
| C | Changes in level of consciousness (seizures, hallucinations, delirium, restlessness, disorientation, anxiety, lethargy, agitation). |
| A | Autonomic dysfunction (diaphoresis, dilated pupils, vomiting, abdominal pain, hyperthermia, tachycardia, diarrhoea). |
| N | Neuromuscular changes (myoclonus, clonus, hyperreflexia, tremor, muscle rigidity). |
Diagnosis
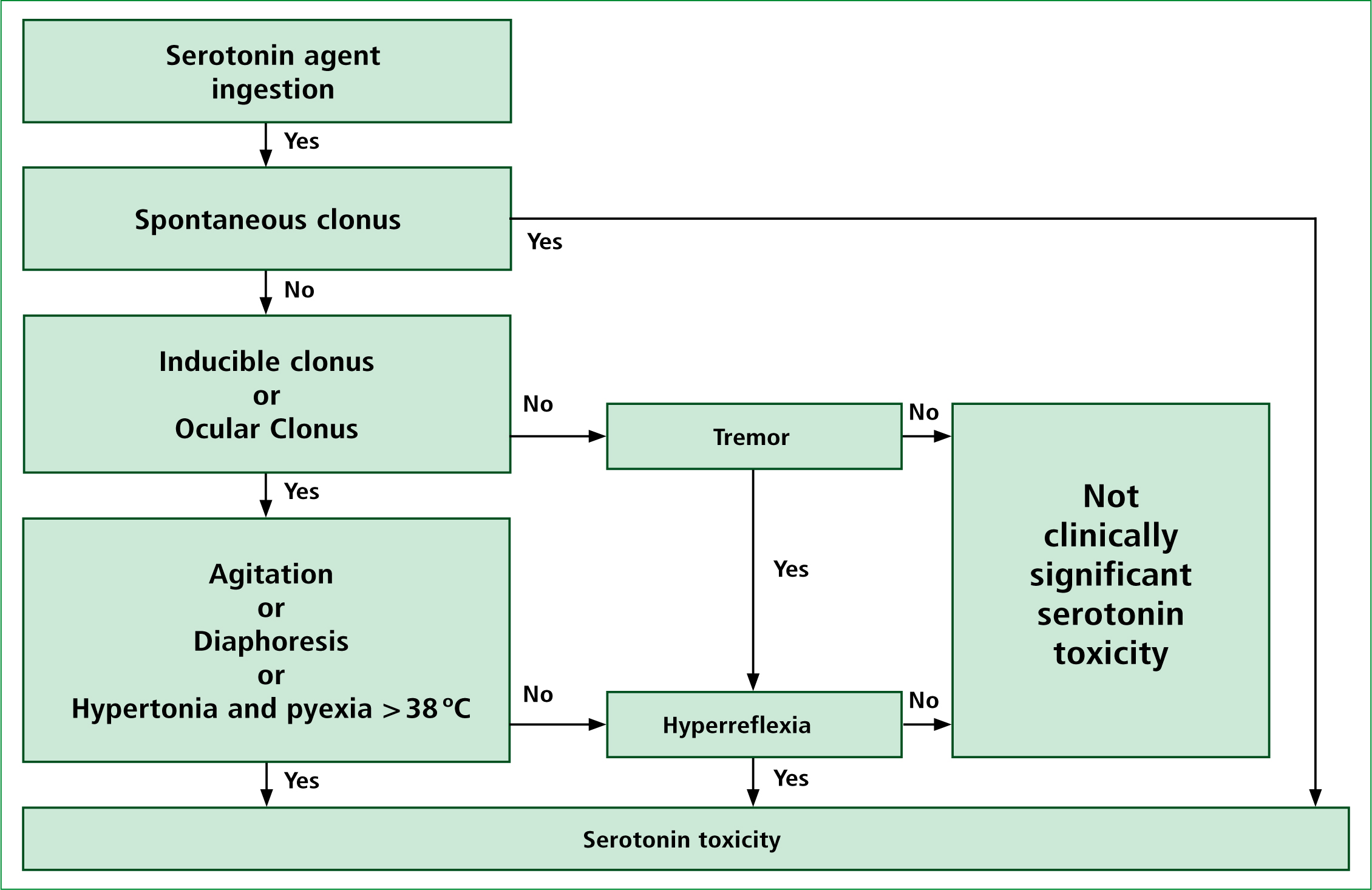
There have been several diagnostic criteria published for the purpose of recognising and diagnosing serotonin toxicity. Sternbach’s (1991) criteria have in the past been the most widely used criteria in the recognition of the condition. The criteria have however been criticised for being non-specific with many other medical conditions having a similar array of signs and symptoms as those suggested by Sternbach. More recently the Hunter serotonin toxicity criteria (HSTC) has been developed by Dunkley et al (2003). This group of Australian researchers retrospectively analysed over 2000 patients who had ingested serotonergic agents and all of whom were diagnosed by a clinical toxicologist as having serotonin toxicity. Dunkley et al (2003) found that there were certain features that were present consistently in many of the cases reviewed. These features included clonus (inducible, spontaneous, or ocular), agitation, diaphoresis, tremor and hyperreflexia. As Figure 2 shows, in the presence of a serotonergic agent the HTSC suggest only spontaneous clonus is needed to reliably diagnose serotonin toxicity. If spontaneous clonus is absent on assessment but inducible or ocular clonus is present combined with agitation or diaphoresis then again the condition can be reliably diagnosed. The HSTC are 84% sensitive and 97% specific and are less likely to miss early or mild forms of serotonin toxicity when compared with Sternbach. With this in mind, the HSTC should be the criteria pre-hospital clinicians use when assessing a patient for possible serotonin toxicity. It is important to remember that in hospital, physicians will rely heavily on patient history and presentation in diagnosing the condition as laboratory tests are of limited value. This makes pre-hospital clinicians equally as able to diagnose the condition as their in hospital colleagues.
It may be argued that pre-hospital clinicians have the distinct advantage of direct visualisation of the scene. This allows them to observe for drug paraphernalia, medication lists, discarded medications and bystander information. This is a unique position to be in and one of great importance. Hospital physicians rely heavily on pre-hospital documentation in order to aid their diagnosis.
Differential diagnosis
There are several differential diagnoses that must be considered when dealing with suspected serotonin toxicity. These include neuroleptic malignant syndrome (NMS), anticholinergic toxicity and malignant hyperthermia. In all three of the conditions patients may display hypertension, tachycardia, tachypnoea and hyperthermia which are also features shared with serotonin toxicity (Boyer and Shannon, 2005).
The clinical manifestations of serotonin toxicity are often misdiagnosed as NMS but can be distinguished based on history and presenting clinical features. NMS develops over days and weeks whereas serotonin toxicity starts showing signs and symptoms around 6 hours after a change in serotonergic medications (Gupta and Nihalani, 2004; Quinn and Stern, 2009). The neuromuscular signs can also distinguish the two conditions with serotonin toxicity displaying neuromuscular hyperactivity whereas NMS produces slow neuromuscular responses (Boyer, 2012). Two of the main features of serotonin toxicity hyperreflexia and myoclonus are unusual in NMS, they are also rare in anticholinergic toxicity. Malignant hyperthermia can occur when individuals are exposed to volatile anaesthetics and depolarising agents and shares many of the characteristics of serotonin toxicity. However, it can usually be ruled out based on careful history taking and the patient not having inhaled any recent volatile anaesthetics ( Litman and Rosenberg, 2005)—Table 3 shows a comparison of the four conditions and their similarities and differences.
| Condition | Time of Onset | Pupils | Skin | Neuromuscular tone | Reflexes | Mental status |
|---|---|---|---|---|---|---|
| Serotonin Toxicity | <12 hours | Mydriasis | Diaphoresis | Increased mostly in lower limbs | Hyperflexia and clonus | Agitation, coma |
| Anticholinergic toxicity | <12 hours | Mydriasis | Red, hot and dry | Normal | Normal | Agitation, delirium |
| Neuroleptic malignant syndrome | 1–3 days | Normal | Pallor, diaphoresis | Rigidity | Bradyreflexia | Stupor, coma |
| Malignant hyperthemia | Up to 24 hours | Normal | Mottled appearance, diaphoresis | Rigidity | Hyporeflexia | Agitation |
Management of serotonin toxicity
There are no randomised controlled trials for the management of the serotonin toxicity. Evidence for the management is based on animal research and human case studies along with expert consensus.
The key to pre-hospital management of serotonin toxicity is the cessation of any serotonergic agent that the patient has been taking. Most cases of serotonin toxicity are self–limiting and clinical features associated with the condition will usually resolve within 24 hours of discontinuing any agent involved (Boyer et al, 2005). The exception to this is those serotonergic agents with long elimination half lives where clinical features may persist for longer than 24 hours. Treatment is mainly supportive and based on the patients presenting clinical features (Isbister et al, 2007). Many cases of mild and moderate toxicity will not require any active treatment from the clinician at scene or on route to hospital but a small minority presenting with severe toxicity may require some form of intervention.
Activated charcoal
Activated charcoal is often used in the treatment of acute ingested poisonings. It is estimated to reduce absorption of poisonous substances up to 70% when given within 30 minutes of ingestion and is usually only beneficial when taken within 1 hour (Roberts, 2002).
The problem presented with serotonin toxicity is that the only known serotonin antagonist used in its treatment is cyproheptadine, which at present can only be administered orally. This would make cyproheptadine less effective in those patients who have been administered activated charcoal. However, current expert opinion is that the administration of activated charcoal may still be warranted if given within one hour (Isbister, 2007).
Oxygen
Oxygen administration will not be required for every patient although should be considered for those patients presenting with hypoxemia. With severe serotonin toxicity truncal muscle rigidity can develop which can impair ventilation causing hypoxemia (Dart, 2004).
For these patients, supplementary oxygen may be of benefit. JRCALC (2009) recommends for acute hypoxemia the administration of 2–6 litres per minute via nasal cannula or 5–10 litres via simple face mask until a reliable SpO2 reading is obtained then maintain 94–98% or a normal range for the patient. If the patient continues to deteriorate due to increasing truncal muscle rigidity intermittent positive pressure ventilation should be considered to maintain adequate ventilation and oxygenation.
Along with this, consideration should be given to using an advanced airway device such as a laryngeal mask airway (LMA) or endotrachael tube if the patient’s condition warrants such an intervention. For those patients with a temperature of over 41.1 °C or impaired ventilation, requesting pre-hospital physician assistance may prove beneficial, as these patients require immediate sedation, paralysis and endotrachael intubation (Frank, 2008; Boyer, 2012).
Benzodiazepines
Evidence has shown that benzodiazepines such as diazepam have an important role to play in the management of hyperthermia and agitation that may develop from serotonin toxicity. Animal models have shown an increase in survival rates from serotonin toxicity when diazepam is administered (Nisijima et al, 2003). Benzodiazepines form an important part of in–hospital treatment of severe serotonin toxicity (Boyer et al, 2005; Isbister et al, 2007). Unfortunately at present this treatment option is not indicated for the pre-hospital environment under JRCALC (2006) guidelines although any seizures should be managed in line with JRCALC guidelines for convulsions in adults and children with diazepam being administered as appropriate.
For those patients who are agitated or combative, again, early consideration should be given for physician support, as early intervention with benzodiazepines such as diazepam and lorazepam can prevent deterioration.
Active cooling
Hyperthermia can occur in severe toxicity and temperatures may reach in excess of 41 °C. Prolonged hyperthermia may lead to multiple organ failure, rhabdomyolysis and death (Pilgrim et al, 2011; Warrick et al, 2012). Animal studies have shown that rapid cooling can reduce the damage caused (Krishnamoorthy et al, 2010). Research on out-of-hospital active cooling for serotonin toxicity is limited but expert consensus suggests it is beneficial and should be undertaken with cooling blankets and ice packs (Nelson et al, 2007). There is no role for paracetamol in the treatment of hyperthermia associated with serotonin toxicity. The hyperthermia is caused by extreme muscle rigidity rather than any hypothalmic alterations to body temperature set point (Boyer and Shannon, 2005).
Fluid resuscitation
Dehydration and hypotension may occur as a result of hyperthermia associated with severe serotonin toxicity. Fluid administration should be considered if hypotension or severe dehydration is present. For heat related illness such as hyperthermia with an absent radial pulse JRCALC (2006) recommends that 250 ml boluses of sodium chloride 0.9% should be administered up to a maximum of one litre with the aim of maintaining a radial pulse. They also recommend for ft individuals that a 250 ml bolus of sodium chloride 0.9% should be commenced before vital signs become abnormal.
Conclusion
Serotonin toxicity is a condition that pre-hospital clinicians will not encounter frequently. Combined with the numerous clinical features, it is a condition that can easily be overlooked. Serotonin toxicity should be considered in any patients presenting with unexplained mental status changes, autonomic hyperactivity or neuromuscular abnormalities. The HSTC should be used when assessing patients with symptoms suggestive of serotonin toxicity as they are currently the most accurate at diagnosing the condition. Most cases will present with mild or moderate symptoms and resolve within 24 hours with no long lasting effects. However a small minority of patients will present with life threatening features which will need to be identified and treated promptly.
