Part one of this series on heat-related illness examined how the disease has been known and written about for thousands of years (Hannides and Walter, 2024).
Since then, there has been some but not much improvement in the understanding of it. Although heat-related illness has been known about for millennia, it still poses a significant threat to humans.
Heat-related illnesses are a spectrum of diseases where heat production exceeds the ability of the body to lose heat, so the core temperature rises. The illness can vary in severity, progressing from heat cramps and heat rash to the more severe heat exhaustion and heatstroke. Heatstroke is a life-threatening disorder usually defined by a core body temperature >40°C with dysfunction of the central nervous system, with symptoms such as delirium, seizures and coma. Heatstroke is described as classic when it results from exposure to environmental heat and exertional when it occurs during strenuous exercise.
Heat-related illness remains in the top three causes of death in athletes (Howe and Boden, 2007); some recent heatwaves are thought to have been associated with over 3000 excess deaths a year in the UK (Office for National Statistics, 2022) and with tens of thousands of deaths worldwide (World Health Organization, 2024), and the incidence is predicted to rise more than 2.5-fold in the next 30 years (Hajat et al, 2014).
There is still therefore much that needs to be learned and understood about the disease to improve survival.
This second article in the series describes the current understanding of heat-related illness and offers a few insights into what may be learnt in the future about how best to manage the condition.
Present day
Effects of heat-related illness
Understanding of the effects of heat-related illness on the body has improved over time.
Some patients develop respiratory or cardiac failure; renal failure may be severe enough to require dialysis, and liver failure may be severe enough to require transplantation (Patel et al, 2023). This is contrary to the assertion of Gallus, the Roman governor of Egypt who led a military campaign in Arabia in 24 BC, who believed that heatstroke ‘attacks the head and … descends to the legs, skipping all the intervening parts of the body’ (Jarcho, 1967).
Immediate complications
Both forms of heatstroke are associated with a risk of death. In patients requiring intensive care with classic heatstroke (CHS), the mortality rate is estimated at 63% (Bouchama et al, 2022); the mortality rate from exertional heatstroke (EHS) patients requiring intensive care is lower, estimated at 27% (Bouchama et al, 2022).
A large systematic review detailed the risk of developing specific signs and symptoms and organ damage in survivors after an episode of CHS (Yezli et al, 2023) (Figure 1)
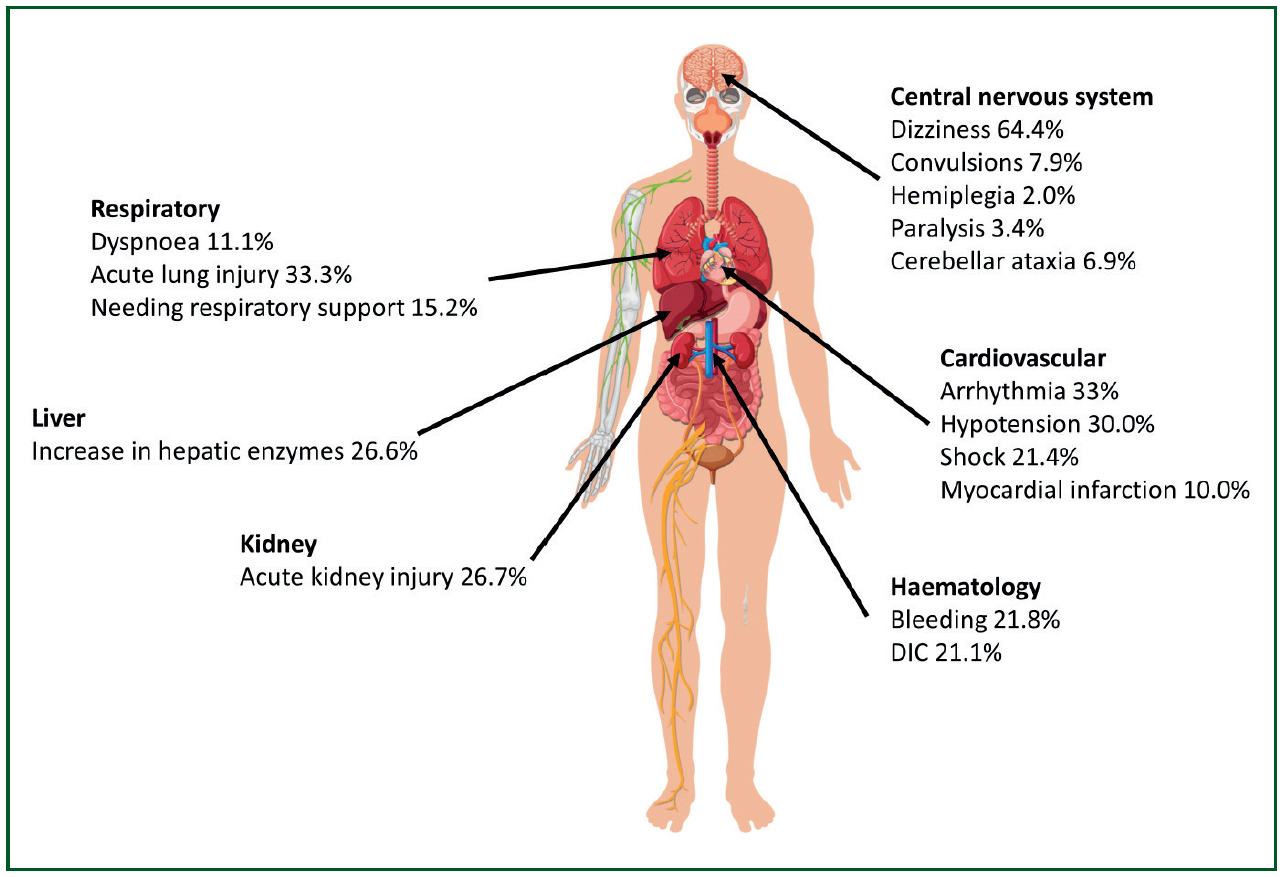
Organ dysfunction may worsen in the first few days following an episode of heat-related illness; however, in most patients, laboratory tests show that organ function tends to return to normal within 2-3 weeks (Ward et al, 2020).
Long-term complications
However, not everyone makes a full recovery. Patients who survive may be at risk of long-term complications.
In one study of patients with CHS, 58% of them died within 28 days, and this had risen to 71% by 2 years (Argaud et al, 2007). After 1 year, the proportion of survivors whose activity levels were severely limited, defined as being unable to work or institutionalised, had risen from 3.7% to 40.7% (Argaud et al, 2007).
Ischaemic heart disease is more likely after heatstroke. One study showed that the risk of developing ischaemic heart disease after heatstroke is 3.5-fold at 12 years (Tseng et al, 2019) and acute myocardial infarction is more than double than in individuals who had not had heatstroke at 14 years (Wang et al, 2019). The risk of heart failure is increased 26-fold at 14 years (Wang et al, 2019).
The long-term risk of developing chronic kidney disease is reported to be 8.8% over 13 years, around twice the normal rate (Tseng et al, 2020).
Persisting neurological signs and symptoms may occur in three-quarters of survivors discharged from hospital, according to one study (Dematte et al, 1998). The cerebellum, which has primarily involvement in balance and coordination, is often affected in cases of persistent neurological dysfunction; this is because the Purkinje fibres in this part of the brain are particularly sensitive to the effects of hyperthermia (Bazille et al, 2005). Damage to many other parts of the brain can also occur (Walter and Carraretto, 2016). Some individuals are left with persistent changes in attention, memory or personality. These may be mild or severe, and can include severe global dementia. Survivors may also be left with physical deficits related to damage to the cerebral cortex, brainstem, spinal cord and the peripheral nervous system. Some survivors remain in a coma or are paralysed (Pease et al, 2009).
Mechanisms of damage
As well as the harmful effects of heat-related illness on the body, our understanding of how organ damage occurs has also improved (Figure 2).
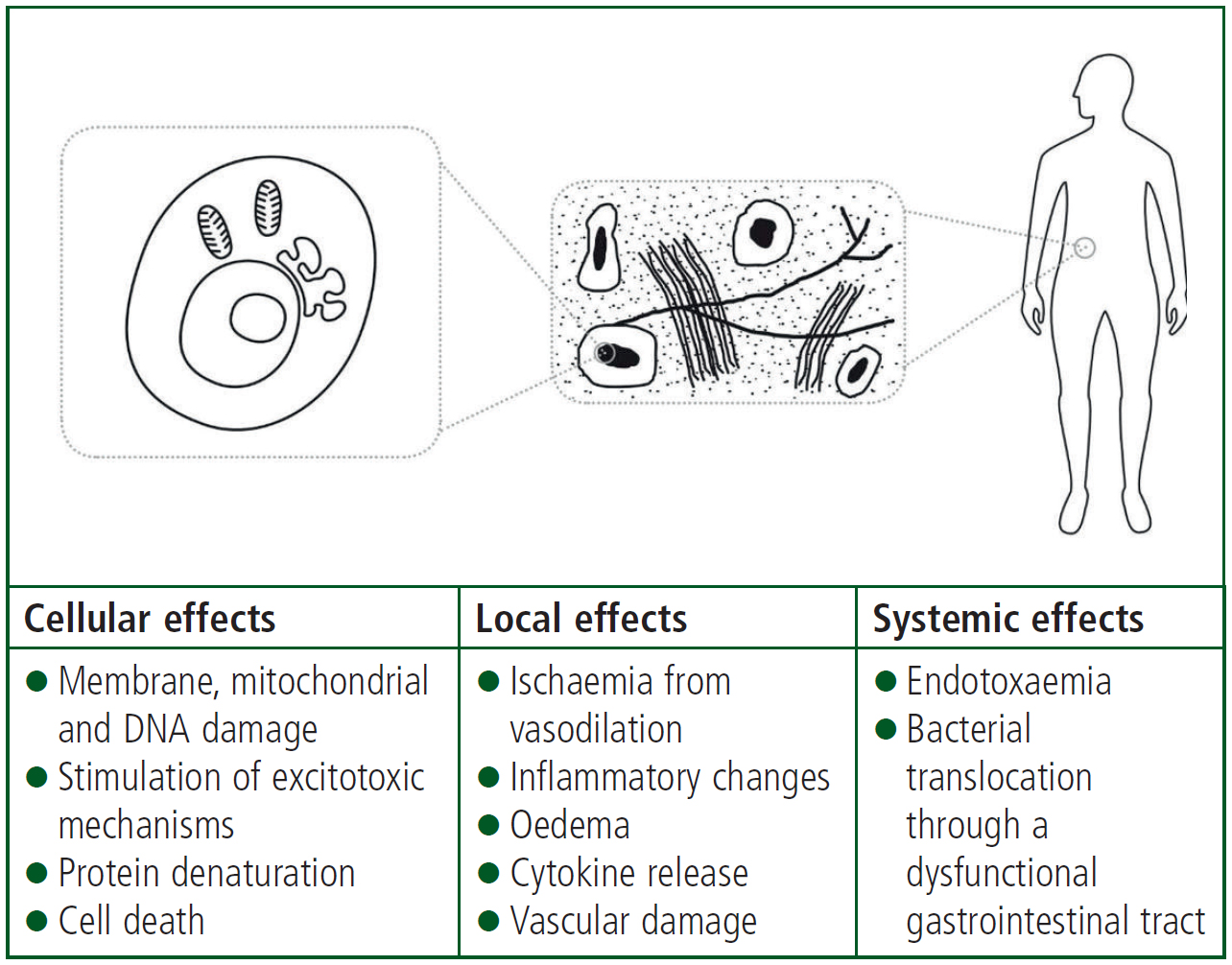
Hyperthermia can damage cells directly. It can affect the stability of the cell membrane and the function of transport proteins in the cell membrane. The synthesis of RNA and DNA in the cells is impaired by exposure to high body temperatures (Hildebrandt et al, 2002).
Direct cell death in humans occurs at temperatures of around 41°C (Hildebrandt et al, 2002). The thermal energy required for cell death is similar to that required for protein denaturation, which suggests that the cell death occurs primarily through the effect of the hyperthermia on protein structure (Hildebrandt et al, 2002). The cells and the environment surrounding them (the interstitium) are at risk of damage from heat after only a short period of time; interstitial changes can occur after only 30 minutes when the core temperature reaches 40.5°C (Vlad et al, 2010).
In addition to the high temperatures directly damaging cells, hyperthermia appears to make the intestinal barrier more permeable. This allows toxins from the gut, such as bacteria and endotoxins, to enter the systemic circulation, discussed further in the ‘Insights into mechanism of heat-related illness’ section of this article.
Treatment
Even if our knowledge of organ failure has advanced, the best treatment is still cooling the patient with water, as Hippocrates suggested around 2500 years ago (Potter, 1923). However, cooling with water is now more refined; we know that this needs to be rapid, and which methods work well.
Physical cooling
Rapid cooling is vital in patients with heatstroke, regardless of the underlying cause. It improves outcome and reduces mortality.
In CHS, mortality rates in those cooled to a core temperature of below 38.9°C within 1 hour of presentation are less than half those of individuals whose temperature remains above 38.9°C (Vicario et al, 1986). If patients with EHS are cooled at a rate greater than 1.5°C every 10 minutes, their risks of death and complications are reduced (Filep et al, 2020). Guidelines therefore often advise cooling the patient on scene before transferring them, unless the patient has a life-threatening condition that necessitates urgent transport to hospital or if cooling is not possible on scene (Belval et al, 2018).
Loss of heat from the body occurs through a number of mechanisms. The majority of heat dissipation— up to 60%—occurs by thermal radiation (Rao and Rajan, 2008). This depends on the difference between the ambient temperature and the temperature of the individual.
Other methods of heat dissipation include convective heat loss via the movement of cooler air across the skin, evaporation of water to vapour from the skin surface (for example from sweating) and exhaling hot gases then breathing in cooler air. Heat can also be lost by conduction to surfaces in direct contact with skin or clothing. A variety of active cooling measures can be used (Figure 3), which employ a number of these mechanisms.
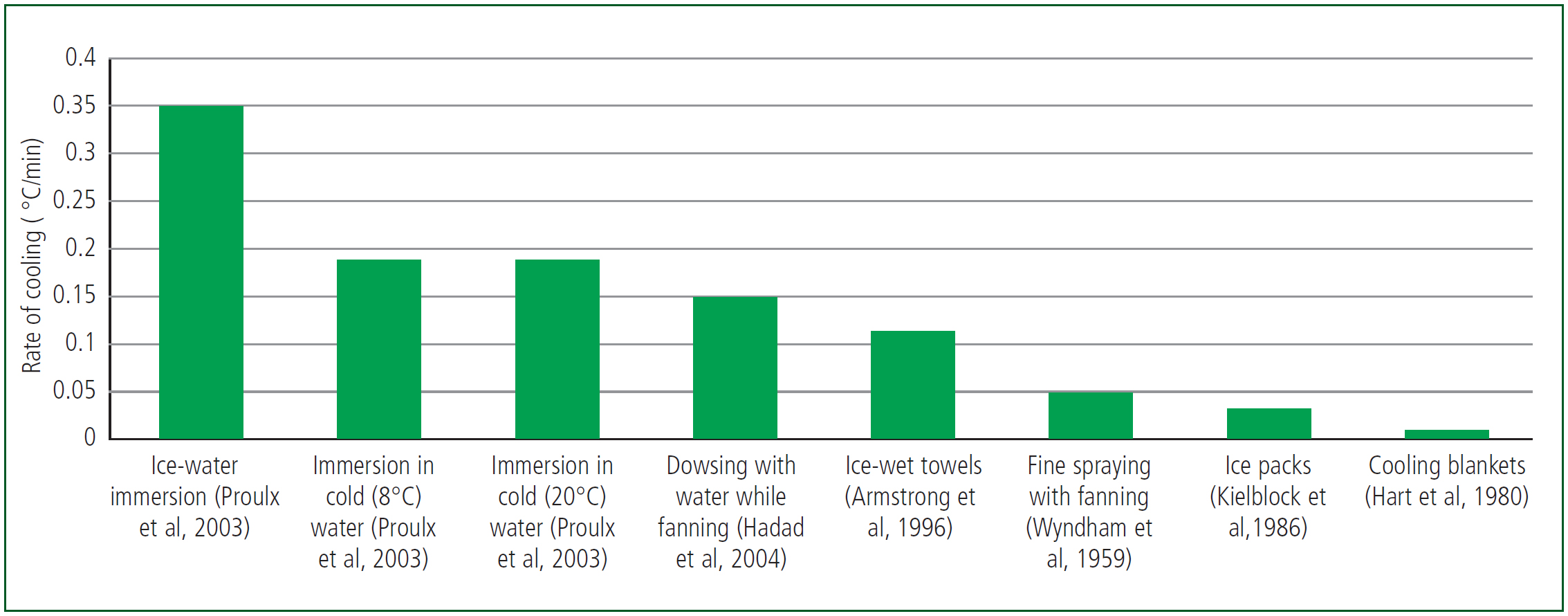
In EHS, cooling by immersion in cold water is the most effective and rapid (McDermott et al, 2009). A more recent method of cooling overcomes some of the physical and logistical challenges of some of the other methods. Tarp-assisted cooling with oscillation (TACO) involves placing the casualty on a waterproof sheet with the edges of the sheet held up by the rescuers. Ice water is placed on the sheet and gently oscillated over the patient. It is effective, with cooling rates of 1.4°C every 10 minutes (Luhring et al, 2016), and has been recommended as the most feasible and effective cooling method for emergency departments (Tucker and Evans, 2023).
Apart from cooling and supportive treatment, no other specific treatment is generally recommended. However, patients may require admission to and treatment in an intensive care unit (Patel et al, 2023).
Drug treatments
Despite the advances in understanding of the pathology behind heat-related illness, no drugs are generally recommended to treat heat-related illness.
Dantrolene is used to treat patients with malignant hyperthermia (MH), a type of severe reaction that occurs in susceptible individuals in response to certain anaesthetic agents. While there are similarities between EHS and MH, dantrolene is not routinely recommended in heatstroke (Hadad et al, 2005).
While antipyretics such as ibuprofen, aspirin and paracetamol may be useful in fevers that result from infection, where the bacteria and viruses stimulate the hypothalamus to raise the temperature, the mechanism of the pyrexia in heatstroke is different. Antipyretics are not useful in heat-related illness and may even worsen the clotting disorders and the liver and renal injury associated with heat-related illness (Grogan and Hopkins, 2002).
Future
Despite the lack of progress in treatments and with the incidence of heat-related illness increasing, there may finally be improvements and progress on the horizon.
Identifying risk factors
American congressman, Benjamin Franklin, believed that ‘an ounce of prevention is worth a pound of cure’. So it may be with heat-related illness.
Numerous factors (Table 1) appear to increase the risk of developing heat-related illness, and identifying these might at least allow susceptible individuals to take extra care when exercising or during hot weather.
| Extrinsic causes | Intrinsic causes | Medications increasing the risk of heat-related illness |
|---|---|---|
| Hot, humid conditions | Low physical fitness levels | Alcohol |
| Heatwaves | Obesity | Amphetamines |
| Lack of acclimatisation to heat | Underlying medical conditions: diabetes, skin disorders, sickle cell trait and cardiovascular disease | Anticholinergic and sympathomimetic agents |
| Cumulative effect of heat exposure | Concurrent infections | Antihistamines |
| Inappropriate clothing | Extremes of age | Benzodiazepines |
| Lack of fans/air conditioning | Male sex | Beta-blockers |
| Disturbed sleep patterns | Calcium-channel blockers | |
| Hydration status | Cocaine | |
| Poor nutrition | Diuretics | |
| Previous episode of heat-related illness | Laxatives | |
| Dehydration | Neuroleptic medications | |
| High levels of physical exertion | Phenothiazines | |
| Genetic predisposition | Thyroid agonists | |
| Tricyclic antidepressants |
Source: Patel et al (2023)
Insights into mechanism of heat-related illness
More is becoming clearer about the ‘peculiar nature’ of heat-related illness, as Gallus described it (Jarcho, 1967).
Some of the problems that develop in heat-related illness are the result of the heat directly damaging tissues and cells. In addition, heat seems to lead to the gut wall becoming more permeable. The tight junctions between gut cells become more permeable, which allows bacteria (Liu et al, 2012) and toxins (Armstrong et al, 2018) from the gut to enter the circulation (Figure 4).
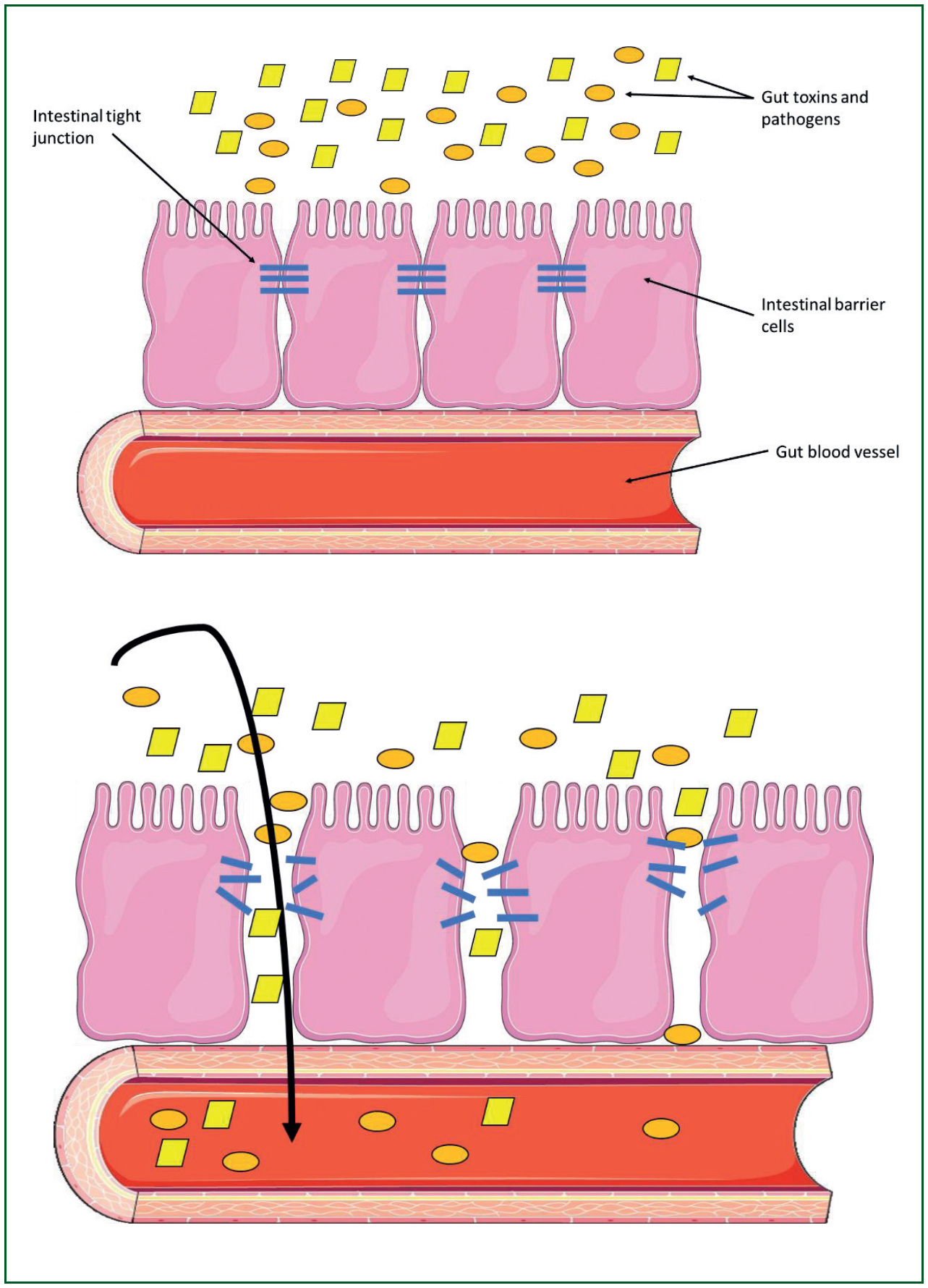
Changes to the intestinal permeability can occur at relatively low core temperatures, around 39°C (Dokladny et al, 2006) and after only a short period of hyperthermia of a few hours (Dokladny et al, 2006).
Increases in intestinal permeability have been observed in humans during EHS (Walter et al, 2021a; 2021b). Some of the gut bacteria release toxic compounds into the circulation (endotoxins or lipopolysaccharides) (Armstrong et al, 2018). Endotoxins and raised lipopolysaccharide levels in the circulation are thought to be responsible for stimulating an inflammatory response in both CHS and EHS (Heled et al, 2013). Cell damage can be caused by inflammation and bacteria in the circulation as well as by the direct effects of a high temperature.
Diet
A number of nutritional components and dietary components, such as vitamins A and D, probiotics and the amino acids arginine and glutamine, appear to improve the function and strength of the intestinal barrier. This raises the possibility of reducing the risk of developing heat-related illness through diet and dietary supplements in individuals who are at high risk.
In one study, taking glutamine supplements 2 hours before exercise in a warm environment reduced several markers of gut leakiness (Pugh et al, 2017). Further research on whether this finding can be applied more widely is needed.
Genetics
Whether an individual is prone to exertional heat illness may in part be because of their genetic make-up. In other conditions where hyperthermia features, such as serotonin syndrome, neuroleptic malignant syndrome and MH, genes play a part (Ortiz et al, 2020), so it might be the case with exertional heat illness (Ren et al, 2019; Protasi et al, 2022).
Exertional heatstroke has a number of clinical and biochemical similarities to MH, and several case reports of patients having both conditions have been published.
Genetic mutations in the ryanodine receptor (RYR), which is involved in mediating cellular calcium release and muscle contraction, account for most cases of MH (McCarthy et al, 2000). Some patients with EHS also display mutations in the RYR1 gene (Roux-Buisson et al, 2016); some authorities therefore advise to consider testing patients with EHS for MH, especially if the EHS is recurrent or if no other obvious cause is identified (Hopkins, 2000).
The genetic basis of EHS may not be as clear as it is with MH; as well as mutations in the ryanodine receptor, mutations in the CASQ1 gene, which codes for the protein calsequestrin, and the TRPV1 gene have been identified (Protasi et al, 2009; Bosson et al, 2020). The calsequestrin protein is found in the sarcoplasm of skeletal muscle and appears to affect the function of the ryanodine receptor. The TRPV1 gene is involved with detecting and sensing changes to the body's core temperature.
Risk of further episodes
Individuals who recover from heat-related illness may be at risk of a future episode. After an episode of EHS, the risk of a further episode has been reported as being over three times higher within the next 2 years (Stearns et al, 2020). Whether this is related to pre-existing genetic, phenotypic or behavioural risks or biochemical or epigenetic changes that develop following the heat exposure is not clear.
Recovery after exertional heat illness
Because recovery can be slow and the risk of a further episode, some authorities recommend that in the 14-21 days following exertional heat illness, an individual is confined to rest and undertakes only essential activities of daily living before progressing to low-intensity activity in cool conditions to ensure exercise can be tolerated (Mitchell et al, 2019; Roberts et al, 2021).
Once an individual can tolerate exercise in cooler conditions, the intensity of exercise can be increased, and exercise can be undertaken in warm as well as cool conditions.
Heat tolerance testing
If exertional heat illness has occurred, heat tolerance testing, where individuals are exercised in a warm and humid environment to assess how quickly their body temperature rises, may give an indication of the risk of developing it again.
There is no standard protocol for such exercise-heat stress tests, and they are not used widely yet. A typical heat tolerance test is that described by Moran et al (2007): participants walk at 5 km/h at a 2% gradient for 120 minutes in conditions of 40°C and 40% relative humidity, 6-8 weeks following an episode of heat-related illness. The test is discontinued and the participant deemed heat intolerant if the individual's core temperature surpasses 38.5°C or the heart rate exceeds 145 beats per minute (Moran et al, 2007).
Potential treatments
The possibility of heat-related illness damaging the gut wall, making it more permeable, and allowing bacteria (Liu et al, 2012) and toxins (Armstrong et al, 2018) from the gut to enter the circulation may offer future opportunities for drug treatments.
Giving antibiotics that are active against gut bacteria appears to have a beneficial effect in animals (Walter and Gibson, 2020a).
Steroids are commonly used to treat inflammatory conditions such as arthritis and Crohn's disease. As some of the damage in heat-related illness results from an inflammatory process, steroids also seem to improve the outcome when they are given to animals with heat stress (Walter and Gibson, 2020b). Research is being carried out to find out if antibiotics and steroids are also useful in humans.
Environment and climate change
Despite improvements in our understanding of how to prevent and treat heat-related illness, higher ambient temperatures and humidity because of climate change are likely to make hyperthermia more likely (Song et al, 2022).
A higher ambient temperature reduces heat loss, as was observed by Homer and Hippocrates. High humidity also reduces the ability to lose heat by evaporation and increases mortality (Barreca, 2012).
More reliable heat indices are therefore probably needed. One example is wet bulb globe temperature (WBGT) measurement, which takes into account humidity, wind speed, and radiant heat in addition to temperature. A WBGT of 35°C, indicating high temperatures and humidity with minimal heat dissipation by both thermal radiation and evaporation, has been suggested as the maximum tolerated temperature; beyond this, hyperthermia will occur (Raymond et al, 2020).
Conclusion
Heat-related illness has been known about for thousands of years, is still common and its incidence is increasing. This is despite the understanding of its risks, mechanisms and treatments being greater than in the past. In the future, genetics may be used to identify who is more at risk, which may bring personalised treatment or prevention. Heat-related illness may catch up with diseases such as coronary artery disease and breast cancer, where personalised or preventive medicine already plays a role. As knowledge of the pathophysiology of heat-related illness increases, treatments other than cooling with cold water, such as antibiotics and anti-inflammatory agents may appear on the horizon. JPP
Key Points
- Heat-related illness is common and its incidence appears to be increasing
- Heat-related illness can cause multi-organ failure and may be fatal
- Some individuals may be more at risk of developing heat-related illness than others; potential future treatments may involve identifying and managing their risk factors
- Survivors of heat-related illness may be at an increased risk of developing chronic heart and renal disease
- Survivors may be more susceptible to a further episode of heat-related illness
CPD Reflection Questions
- Is it possible to identify individuals who may be more at risk of heat-related illness than others?
- What is the best way to manage a patient with heat-related illness?
- Are there any medications that are likely to be useful in treating heatstroke?


