Travelling to high-altitude regions ≥2500 m above sea level requires physiological adaption and acclimatisation to a lower partial pressure of oxygen (Higgins et al, 2010; Shen et al, 2012). If adaption processes fail owing to rapid ascent or exceptional physiological characteristics (e.g. chronic obstructive pulmonary disease (COPD), heart failure), individuals are at risk of acute mountain sickness (AMS) and the development of high-mortality illnesses including high-altitude cerebral oedema and pulmonary oedema (Higgins et al, 2010; Imray et al, 2010; Jensen and Vincent, 2020).
Globally, AMS affects between 40% and 90% of unacclimatised individuals ascending over 500 m per day to altitudes between 4500 m and 6000 m (Muza et al, 2010; Kayser et al, 2012). AMS symptoms commonly include headache, loss of appetite, nausea, dizziness, insomnia and lassitude, and regularly present 4–12 hours after arrival at the high-altitude location (Luks et al, 2019; Swenson and Bärtsch, 2016). Importantly, AMS symptoms resolve with descent or supplementary oxygen (Higgins et al, 2010; Jensen and Vincent, 2018).
Timely and accurate prehospital assessment is essential for the successful prediction of AMS (Karinen et al, 2010). If those with or in the process of developing AMS could be identified more efficiently, treatment could be prescribed, thus reducing the likelihood of a rescue effort or further physiological deterioration (Luks et al, 2019).
In remote, high-altitude environments, paramedics, as health professionals, often play a critical role in the prediction of AMS development among travellers (Luks et al, 2019). Currently, the Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness (2010) recommend that paramedics use pulse oximetry and the Lake Louise acute mountain sickness score (LLS) (Roach et al, 2018) to assess patients efficiently and non-invasively in the field (Karinen et al, 2010; Wagner et al, 2012; Roach et al, 2018). The accuracy of pulse oximetry in predicting AMS is unclear so requires further research.
Aims
The primary objective of this review is to establish the effectiveness and accuracy of pulse oximetry as a predictive tool for identifying the development of AMS during ascent to high altitudes.
Methods
This literature review employs the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method (Figure 1) (Page et al, 2021). A search of the Medline database was conducted between October 2020 and January 2021 using the keywords ‘SpO2’, ‘pulse oximetry’, ‘AMS’, ‘acute mountain sickness’ and ‘high altitude’. Additional primary research was identified within sources cited in meta-analyses, review articles and consensus guidelines. Primary research and meta-analyses were included, and letters to the editor, case reports, review articles, consensus guidelines and grey literature were excluded. Finally, the following additional inclusion criteria were applied: full-text availability; peer review; and published between 2010 and 2020.
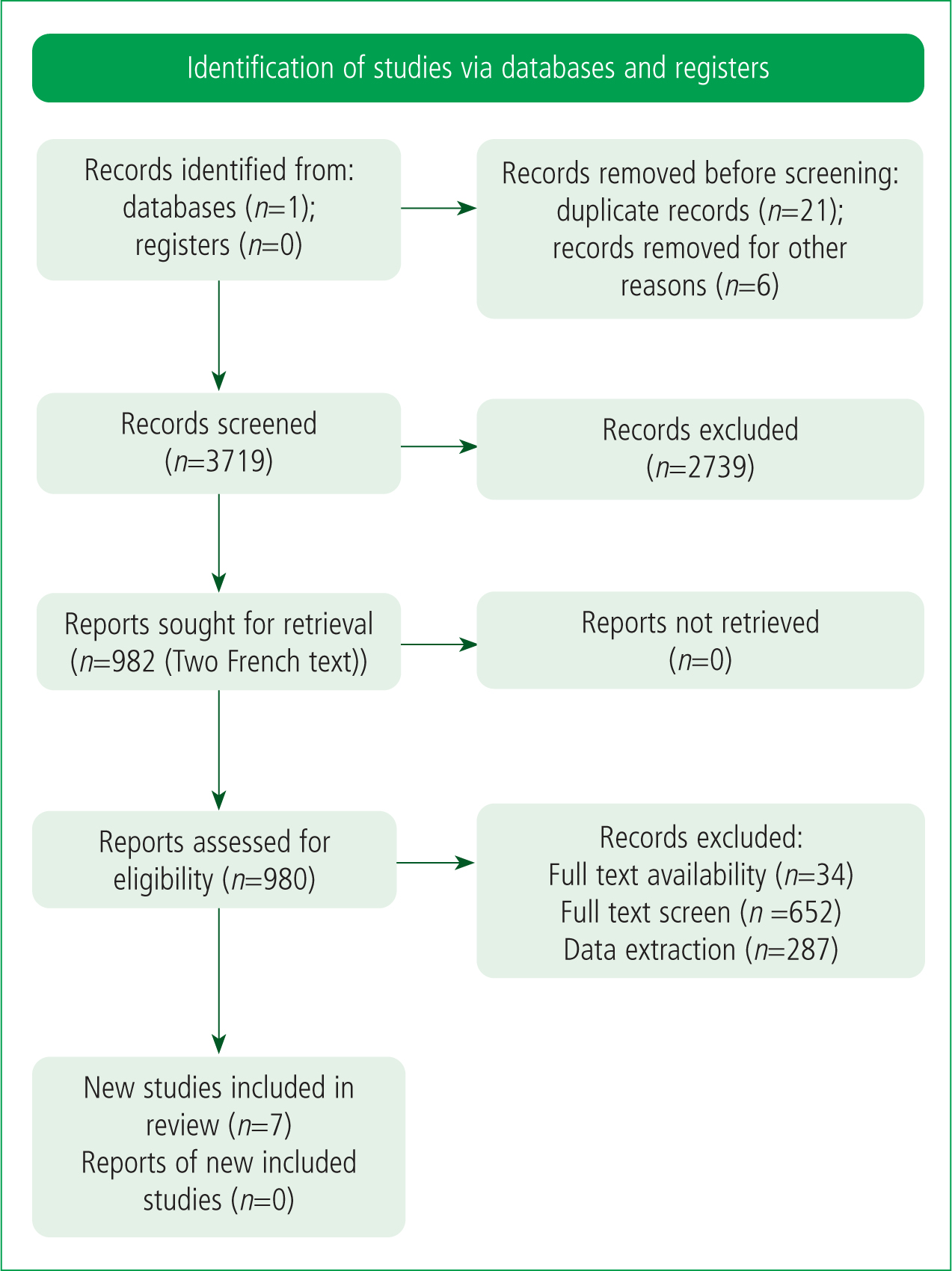
Results
Using the above search strategy, one prospective single-centre study, three prospective observational studies, one retrospective cohort multicentre study and two observational retrospective single-centre studies were identified. Three consensus statements and two systematic literature reviews were also noted but subsequently discarded. The studies included for qualitative analysis are presented in Table 1.
| Author | Title |
|---|---|
| Chen at al (2012) | Change in oxygen saturation does not predict acute mountain sickness on Jade Mountain |
| Karinen et al (2010) | Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent |
| Leichtfried et al (2016) | Diagnosis and prediction of the occurrence of acute mountain sickness measuring oxygen saturation—independent of absolute altitude? |
| Faulhaber et al (2014) | Resting arterial oxygen saturation and breathing frequency as predictors for acute mountain sickness development: a prospective cohort study |
| Tannheimer and Lechner (2019) | The correct measurement of oxygen saturation at high altitude |
| Ross et al (2013) | Measuring arterial oxygenation in a high-altitude field environment: comparing portable pulse oximetry with blood gas analysis |
| Milner and Mathews (2012) | An assessment of the accuracy of pulse oximeters |
Does pulse oximetry predict onset of acute mountain sickness?
Pulse oximeters commonly record oxygen saturation (SpO2) and pulse rate. SpO2 is a percentage measurement of oxygen-carrying haemoglobin relative to the total arterial haemoglobin; measurements are typically taken from peripheral capillary beds such as fingertips (Pedersen et al, 2014). Normal SpO2 in adults at sea level is 94–98% (Pedersen et al, 2014). At high altitude, pulse oximetry is a practical method of obtaining SpO2 measurements owing to its non-invasive, portable nature (Luks and Swenson, 2011).
Chen et al (2012) conducted an observational prospective cohort study that examined the correlation between resting oxygen saturation (SpO2) and AMS during an ascent of Jade Mountain in Taiwan. To establish the presence and severity of AMS, participants self-completed an LLS questionnaire (Roach et al, 2018) and used the Oximax handheld pulse oximeter (Oximax N65, Nellcor Puritan Bennett Ireland, Mervue, Galway, Ireland) to measure SpO2. Testing was overseen by a health professional.
Of the 1132 eligible participants, 787 completed the study. Subjects were assigned in a 7:3 ratio to either a hypothesis-testing group (HTG) (552/787) or a validation group (VG) (235/787). The HTG participants had AMS, and the VG subjects did not have AMS. On average, individuals with AMS had a lower SpO2 than those without (6.24%±0.21% vs 7.39±0.31% respectively; P=0.002). However, the sensitivity (56.59%) of this finding was low, indicating a high risk of bias; consequently, it was concluded that SpO2 is a poor predictor of AMS development.
Chen et al (2012) study's examination of ‘real-world’ pulse oximetry and LLS application in a high-altitude field setting shows high external validity and a direct application of SpO2 to the prediction of AMS during ascent to high altitude (Fink, 2014; Kite and Whitley, 2018). However, data were collected from a single participant group on one date in October 2012, thus limiting the validity of both internal and external findings.
In contrast to Chen et al (2012), Karinen et al (2010) identified a significant correlation between SpO2 and AMS, performing a retrospective cohort study to investigate the differences in SpO2 between subjects who had (AMS+) or had not (AMS−) developed AMS at altitudes between 2400 m and 5300 m. They included 83 ascents to high altitudes made by 73 individuals in Denali (Alaska), Shisha Pangma (Tibet), Mount Everest Base Camp (Nepal), Island Peak (Nepal) and Ulugh Muztagh (Tibet). Participants took pulse oximeter readings at rest and at 3500 m, 4300 m and 5300 m. The authors reported that 47% of participants had AMS between 2400 m and 5300 m. The mean SpO2 values of the AMS−group at 3500 m, 4300 m and 5300 m were 7.4%, 7.8% and 7.7% higher than the mean SpO2 values of the AMS+ cohort respectively. These findings suggest that a reduced SpO2 between 3500 m and 5300 m corresponds to an increased risk of developing AMS.
The study methodology also demonstrates good result generalisability of findings given the broad distribution of the locations included. This geographic diversity is an improvement on the single site studied by Chen et al (2012). It can therefore be argued that the Karinen et al (2010) study offers a superior demonstration of pulse oximeter utility for AMS detection.
However, a retrospective methodology was employed, which is susceptible to recall bias, as the authors attempted to correlate historical data with a hypothesis, thus limiting the validity of their findings. Furthermore, the work is liable to participant bias as people in athletic groups are often reluctant to admit injury or illness so as not to appear weaker than other members (Haslam et al, 2012). This study included small cohorts of experienced mountaineers, and participants may have felt psychosocial pressures to hide symptoms of AMS so as not to appear feeble. Consequently, there is a risk of data collection bias from false reporting, which limits validity and reliability (LoBiondo-Wood and Haber, 2014).
Leichtfried et al (2016) conducted a retrospective study that investigated the relationship between AMS and SpO2 in participants ascending to a high altitude. Volunteer participants (n=204) climbed to altitudes of between 2500 m and 5500 m in the Himalayas (71.6%), South America (7.5%) or Africa (13.9%). This study showed a weak-to-moderate but significant relationship between declining SpO2 and associated increased AMS prevalence (Spearman rank correlation coefficient = −0.370). These data correspond with results from Karinen et al (2010), thereby improving the reliability of both data sets (Heale and Twycross, 2015). However, despite this consensus, both publications are inconsistent with the findings of Chen et al (2012). Moreover, the Chen et al (2012) study sample size is 583 participants larger than that evaluated by Leichtfried et al (2016) so the margin for error is likely to be smaller and mean values significantly more accurate and reliable.
Faulhaber et al (2014) performed a prospective cohort study (n=55) and proposed that resting SpO2 and breathing rate are direct predictors for the development of AMS. They recorded AMS symptoms at 3, 6, 9 and 12 hours after a 20-minute exposure to 4500 m altitude equivalence in a normobaric chamber. Individual participant data were compared against three hypothesis models, each applied after 30 minutes of exposure to 4500 m. AMS is predicted in models 1 (M1), 2 (M2) and 3 (M3) when regression equations for altitude-dependant mean SaO2 values of AMS-susceptible (SaO2-suscept) and AMS-resistant (SaO2-resist) persons equal SaO2 <(SaO2-suscept + SaO2-resist) / 2 (e.g. SaO2<81.2 at 4500 m), SaO2 ≤SaO2-suscept (e.g. SaO2 ≤79.0% at 4500 m) and SaO2 <SaO2-resist (e.g. SaO2 <83.4% at 4500 m) respectively; this model is based upon Burtscher et al (2004). The study determined that M1, M2 and M3 identified AMS development in 62%, 67% and 69% of cases respectively (Faulhaber et al, 2014).
These findings correlated with those of Leichtfried et al (2016) and Karinen et al (2010) in identifying a predictive correlation between SpO2 and AMS development. However, Faulhaber et al (2014) reported data only from an altitude of 4500 m, unlike Leichtfried et al (2016) and Karinen et al (2010), who investigated a range of altitudes. The disparity in elevations limits the generalisation of the findings from the former in comparison to the data from the latter two studies (Branch and Pennypacker, 2013).

A second limitation is that Faulhaber's group conducted their observations in a simulated normobaric chamber. In a systematic literature review, Coppel et al (2015) identified physiological differences between hypobaric and normobaric environments, so comparability is limited. Moreover, Millet et al (2012) suggest that AMS incidence is different in the two pressure contexts. Since Faulhaber et al (2014) did not perform their study in either a hypobaric or high-altitude environment, external validity and generalisability of the findings are limited (Branch and Pennypacker, 2013; Heale and Twycross, 2015).
Pulse oximetry accuracy at high altitude
A pulse oximeter is a non-invasive diagnostic tool used routinely in high-altitude medicine to measure SpO2 (Luks et al, 2019). Since the parameter is heavily influenced by factors including temperature, irregular breathing patterns and subjective SpO2 value interpretations, contemporary researchers have investigated the extent to which pulse oximeters provide an accurate measurement of SpO2 at high altitude (Luks and Swenson, 2011; Basnyat, 2014; Luks et al, 2019).
Tannheimer and Lechner (2019) conducted a retrospective study to evaluate whether visually interpreted mean SpO2 matched computer-generated mean SpO2 at an altitude of 6013 m. Participants performed their measurements using a PalmSat2500 (Nonin, Plymouth, Minnesota, US) placed on a fingertip. SpO2 measurements were taken visually over three minutes and compared against an automated mean generated by readings taken every 4 seconds over the same time period. The study showed a strong correlation (R2=0.98; P<0.0001) between visual mean SpO2 and automated mean SpO2. This relationship suggests that visual SpO2 interpretation is highly accurate.
One notable strength of the above study is that participants were experienced in pulse oximetry and had received training at an altitude of 3372 m before the study started. The data set was potentially more accurate and reliable as participants were practically competent at measuring and recording SpO2. Despite this strength, the study sample size of just four participants dramatically limits reliability (Hackshaw, 2008). Moreover, as all participants were experienced, generalisability of data to laypersons or comparison with studies that recruited only lay people is not possible. A further limitation is the lack of a control group, which limits the internal validity of the data set as the findings could be neither proved nor disproved (Parahoo, 2014).
Ross et al (2013) overcame this limitation in a quantitative volunteer study by comparing pulse oximetry to arterial blood gas (ABG) data. They appraised the accuracy of two probe devices, the Nonin PalmSAT 2500 and the Nonin Onyx 9500, and five probe attachments, namely the Nonin PalmSAT 2500 device, the Nonin PalmSAT 8000AA, the 8000SM finger probe, the Nonin PalmSAT 8000Q ear probe and the Nonin PalmSAT 8000R forehead probe, in comparison to ABG. The PalmSAT 8000SM finger probe was the most accurate and exhibited a mean difference of 2.17% with a standard deviation of 2.56% (95% CI [1.42–2.92]) compared with ABG. These authors concluded there was no statistical difference between the accuracy of pulse oximetry and ABG at an altitude of 2100 m; this is in keeping with the data presented by Tannheimer and Lechner (2019).
The main limitation was that only one brand of pulse oximeter was used, which resulted in a narrow data set. It could be argued that Tannheimer and Lechner (2019) suffered the same limitation. As only the Nonin pulse oximeter was used in both studies, the significance of the comparison is limited and the generalisability of findings reduced.
In contrast to the above two studies, which concluded in favour of the accuracy of pulse oximeters, Milner and Mathews (2012) found notable limitations. In a quantitative volunteer study, these researchers used a Lightman spectrometer to investigate the emission spectra of the light-emitting diodes in 847 pulse oximeters in 29 UK NHS hospitals. It was determined that 10.5% (89/847) had a fault so produced inaccurate measurements; these oximeters were excluded from further investigation. Of the remaining devices, 23.3% (169/758) had an error that caused an inaccurate SpO2 measurement of >4% between SpO2 readings of 70–100%; 9.2% (70/758) produced a measurement error of <4% between 80% and 100%. In total, 258 of the 847 pulse oximeters failed to perform to the manufacturer's specifications, raising significant concerns regarding the accuracy of portable pulse oximeters.
Milner and Mathews (2012) were, however, unable to identify the age of the pulse oximeters included. They recognised that pulse oximeter accuracy will decline over time and that other studies, such as that by Tannheimer and Lechner (2019), used new equipment. Therefore, no statistical comparison is feasible and external validity is limited (Heale and Twycross, 2015). Furthermore, Milner and Mathews (2012) did not collect data at high altitude, which limits the generalisation of findings to high-altitude research.
Limitations
One limitation of this review is that eight of the nine critiqued studies were international. This limits their external validity and application to UK practice. One study investigated pulse oximetry in a hospital setting at a low altitude. This study was included in the review as the research was primary and applicable to pulse oximeter accuracy at high altitude. The external validity of this review is not impaired by the inclusion of this study.
The other limitation is the small diversity of methodologies used within the critiqued literature. All the research is quantitative and uses a volunteer or cohort experimental design, which increases the risk of data collection bias and limiting the reliability and validity of interpretation.
Despite these limitations, the variation in study locations and sample sizes dramatically strengthen the generalisability of this review's conclusions.
Conclusion
In the prehospital high-altitude environment, the ability to predict and recognise AMS is essential to reducing altitude-related mortality and morbidity. This review has analysed and critiqued a number of studies relating to the use of pulse oximetry in this context for the prediction of AMS, as well as the accuracy of pulse oximetry as a diagnostic tool. Several contemporary sources of literature demonstrate the association of declining SpO2 with AMS. However, there is no compelling evidence to suggest that SpO2 independently predicts or correlates with AMS development.
The accuracy of pulse oximetry in this review is inferred from both high-altitude and sea-level studies. The former showed that both clinical and non-clinical individuals are able to accurately interpret pulse oximeter readings. However, in a more extensive in-hospital study at sea level, the accuracy of the pulse oximeter per se was criticised. In summary, the evidence supports the concept that pulse oximeters are a useful and accurate tool if they have been tested adequately before use.
This review has also identified limitations in the literature investigating the relationship between SpO2 and AMS. First, the accuracy of pulse oximetry is shown to decline in cold environments and when SpO2 is below 80% (Ross et al, 2013). Thus, given the cold climate of a high-altitude environment, studies conducted at a high altitude have a high probability of external bias.
The evidence suggests that the use of pulse oximetry at high altitudes provides more benefit than harm to individuals but this review does not support the use of SpO2 as an independent predictor of the presence or incipient development of AMS.
Recommendations
This review has identified an opportunity for further research into the accuracy of pulse oximetry at high altitudes and its relationship to AMS via the systematic study of SpO2 and AMS development at incrementally increasing heights.
Moreover, there is a gap in the literature for a qualitative study that correlates participant perceptions of AMS symptomatology with their actual SaO2 measurement.
There is no literature that investigates the emission spectra of light-emitting diodes in pulse oximeters at high altitude. This is essential to the determination of pulse oximetry accuracy in this environment.

