Despite advances in pain medication and widely accepted guidelines for the treatment of pain, such as the World Health Organization (WHO) analgesic ladder (Ventafridda et al, 1985), inadequate assessment and management of acute pain remains common in prehospital and emergency department (ED) settings (Guéant et al, 2011; Dale et al, 2015; Pierik et al, 2015). Poor or slow pain management practices can have a negative impact on patient care, patient-reported outcomes and patient flow (Thomas, 2013).
Methoxyflurane belongs to the fluorinated hydrocarbon group of volatile anaesthetics (Tomlin et al, 1965). As with all anaesthetics, the precise mechanism of action is unclear. Methoxyflurane is unique in this anaesthetic family in having well-documented analgesic properties at low doses (Blair et al, 2016; Jephcott et al, 2018).
It has been used extensively for more than 30 years in Australasia as a self-administered, rapidly acting analgesic agent for short-term pain relief in emergency medicine, as well as for minor surgical and dental procedures (Medical Developments International, 2016). In the UK and Ireland, methoxyflurane is indicated for the emergency relief of moderate-to-severe pain in conscious adult patients with trauma and associated pain (Electronic Medicines Compendium (eMC), 2018).
The Penthrox® inhaler (Galen Limited, Craigavon) (Figure 1) is a green, whistle-shaped, single-use device that delivers one analgesic dose of methoxyflurane per pack (eMC, 2018). Each pack contains 3 ml methoxyflurane; the maximum recommended dose in 24 hours is two 3 ml vials and no more than 5 vials (15 ml) in a week; additionally, it is not for use on consecutive days (EMC, 2018).
Methoxyflurane gained popularity in the early 1960s as an inhaled anaesthetic agent. However, following extensive use, reports of nephrotoxicity (kidney damage) emerged (Crandell, 1965). Methoxyflurane, a fluorinated hydrocarbon, was shown to cause nephrotoxicity in a dose-dependent manner. High doses of methoxyflurane, as a result of a long duration of anaesthesia, resulted in high serum fluoride levels and corresponding fluoride-mediated nephrotoxicity (Cousins and Mazze, 1973). As a consequence, the drug declined in popularity for general anaesthesia; however, it was noted that it exhibited excellent analgesic properties in much lower doses than those used for anaesthesia (Cousins and Mazze, 1973).
The upper limit of safety for methoxyflurane has been determined to be 2 MAC (minimal alveolar concentration)-hours (Dayan, 2016). Doses below 2 MAC-hours have not been associated with nephrotoxicity. Penthrox contains 3 ml methoxyflurane per pack, which is equivalent to 0.3 MAC-hours. The maximum daily dose of methoxyflurane is 6 ml, which equates to 0.59 MAC-hours (Dayan, 2016; Medicines and Healthcare Products Regulatory Agency (MHRA), 2017). Patients are limited to two doses of Penthrox (2x3 ml) in a single episode (EMC, 2018) to ensure exposure to methoxyflurane is kept well below the safe upper limit. It is accepted that the recommended low dose of methoxyflurane is not associated with nephrotoxicity (Dayan, 2016).
Methoxyflurane is an inhaled agent so a patient will exhale some of the drug and occupational exposure can occur. A recent occupational exposure assessment determined that the maximum exposure level (MEL) for methoxyflurane is 15 ppm, expressed as an 8–hour time-weighted average (Frangos et al, 2016). This MEL is ≥50 times higher than the mean observed time-weighted average exposure for paramedics and medical personnel involved in supervising the administration of methoxyflurane (Frangos et al, 2016). It is ≥10 times higher than the modelled time-weighted average of typical adequately ventilated treatment settings such as ambulances and treatment rooms (Frangos et al, 2016). An activated carbon (AC) chamber is included with every Penthrox pack (eMC, 2018) in the UK and Ireland, which adsorbs exhaled methoxyflurane. However, occupational exposure studies conducted in ambulance personnel in Australia have shown that, even in the absence of this AC chamber, the mean measured occupational exposure levels were significantly below the MEL (Frangos et al, 2016).
Despite a large volume of published literature supporting the efficacy and safety of methoxyflurane at analgesic doses (Blair et al, 2016; Jephcott et al, 2018), most studies have been observational and uncontrolled. However, two large randomised, controlled trials (RCTs) have compared methoxyflurane with placebo (Coffey et al, 2014) and with intramuscular (IM) tramadol (100 mg) (Lim et al, 2016). In the placebo-controlled RCT, methoxyflurane was found to deliver effective pain relief to 87.2% of patients, with approximately half of them reporting first pain relief within 1–5 inhalations (Coffey et al, 2014). Compared with IM tramadol delivered in the prehospital setting by ambulance personnel (i.e. paramedics), methoxyflurane resulted in a significantly greater reduction in patient pain scores, with a faster time to onset of pain relief (Blair et al, 2016; Lim et al, 2016). Methoxyflurane was also quicker to prepare and administer than IM tramadol (Blair et al, 2016; Lim et al, 2016). Additionally, its use in a search- and-rescue setting has been reviewed, where it was found to be a safe and efficacious analgesic (Griffiths, 2017).
The purpose of this case series is to examine the efficacy and safety of methoxyflurane (Penthrox) in the prehospital setting.
Study setting
These case reports are from prehospital settings and involve patients experiencing traumatic injuries who were attended by rapid response doctors. The cases are from the UK and Ireland.
Method
Doses of 3 ml methoxyflurane (Penthrox) were delivered using the ‘green whistle’ system (Figure 1), which allowed patients to self-administer an inhalatory analgesic in cases of moderate-to-severe pain from acute trauma. A second 3 ml dose could be administered by the responding doctor after the first dose had been finished, if needed. If analgesia was not sufficient after 1 minute, the patient could be advised to cover the entrainment hole on the top of the AC chamber with their finger while inhaling through the device to increase the dose of methoxyflurane being inhaled. Additional or alternative analgesia could be provided to patients as needed.
Methoxyflurane was administered and pain score assessments were undertaken by one of two senior clinicians associated with the anaesthesia, trauma and critical care (ATACC) prehospital care team while responding to 999 calls for traumatic injury in the UK and Ireland. Information collected at the scene included details of the mechanism of injury, details of the patient, their visual analogue scale (VAS) pain score (0=no pain to 10=worst pain imaginable) initially and at 1 minute, 2 minutes and 5 minutes after treatment. Confirmation of patient injuries was added later; this was obtained from the relevant hospital clinician.
Statistics
Data were analysed using StatsDirect version 3.1.18 for tests of normality for sample data (Shapiro-Wilk test), charts and summary statistics. R version 3.5.0 was used for statistical modelling and model checking. A linear mixed-effect model (lme function from R, nlme package) was chosen, with the times of VAS testing and the presence/absence of polytrauma specified as fixed effects and individual patients as a random effect. Choosing this type of model addresses the lack of independence of repeated measurements on the same patient over time. The level of significance was set at 5%. A maximal model was fitted first, which included interaction between parameters, polytrauma as a fixed effect and a correction for autocorrelation. The model was refined until the minimal adequate model was identified. Models were compared in the standard way using the anova function in R.
VAS scores for pain were plotted as time series graphs with 95% confidence intervals for the polytrauma and single injury groups. Time 0 was defined as the pre-administration VAS score.
Results
Fourteen patients (78%) were men and four (22%) women. The age range was 19–84 years and the mean age, 48 years; the mean age of the male patients was higher than that of female patients, at 50.4 years compared with 40.5 years.
Road traffic collisions (RTCs) accounted for the majority of injuries (seven patients), with falls from greater than 2 metres and crush injuries accounting for three patients each; falls from less than 2 metres and sporting injuries accounting for two patients each; and there was one patient with a burn injury. Half (50%) of patients sustained single-system injuries (e.g. ankle fracture or shoulder dislocation) and the other 50% experienced multisystem injuries (e.g. head, chest and pelvic injuries). An overview of the incidents and pain scores is given in Table 1; more detail on these incidents can be obtained from the authors.
| Incident | Casualty (sex, age) | Injuries | Baseline pain score (VAS) | Pain score (VAS) at 1 minute | Pain score (VAS) at 2 minute | Pain score (VAS) at 5 minute |
|---|---|---|---|---|---|---|
| RTC | Male, 76 | Multiple rib fractures, small pneumothorax | 6 | 1 | 1 | 1 |
| RTC | Male, 53 | Multiple lower limb fractures, acetabular fracture, mesenteric bleeding | 10 | 8 | 3* | 3 |
| RTC | Male, 69 | Clavicle fracture, rib fracture, bruised sternum | 9 | 3 | 3 | 1 |
| RTC | Female, 72 | Cerebral contusion, spinal fracture (C2), bilateral flail chest, lung laceration, pneumothorax | 10 | 2 | 2 | 2 |
| RTC | Male, 56 | Humerus fracture, rib fracture, lumbar facet fracture | 9 | 3 | 2 | 2 |
| Fall | Female, 45 | Bimalleolar fracture | 8 | 3 | 1 | 1 |
| Weight-lifting injury | Male, 19 | Anterior shoulder dislocation | 9 | 2 | 2 | 2 |
| Fall | Male, 60 | Anterior shoulder dislocation | 9 | 4 | 4 | 2 |
| Cow attack | Male, 63 | Multiple rib fractures, bilateral lung contusions, small liver tear | 8 | 1 | 1 | 1 |
| Fall | Female, 20 | Muscle spasm – background chronic back pain | 10 | 4 | 4 | 4 |
| Fall | Male, 44 | Wedge fracture (L2), multiple rib fractures | 7 | 4 | 4 | 4 |
| RTC | Male, 36 | Multiple rib fractures | 8 | 3 | 3 | 2 |
| Entrapment in combine harvester | Male, 32 | Amputation across wrist | 10 | 4 | 4 | N/A (patient intubated) |
| Sports injury | Male, 35 | Dislocated patella | 8 | 3 | 1 | 1 |
| Injury while tying shoelace | Male, 84 | Dislocated hip | 8 | 2 | 2 | 2 |
| Fire | Male, 23 | 25% burns | 10 | 7 | 5 | 5 |
| RTC | Female, 25 | Open disarticulated knee, minor glass wounds | 9 | 6 | 6 | 6 |
| Crush injury | Male, 56 | Multiple rib fractures, dislocated shoulder, small pneumothorax | 9 | 3 | 2 | 2 |
VAS: visual analogue scale; RTC: road traffic collision Pain scores were taken using the 10-point VAS pain score, with 0 being no pain and 10 being the worst pain imaginable. Pain scores were taken at 1 minute, 2 minutes and 5 minutes after administration of methoxyflurane.
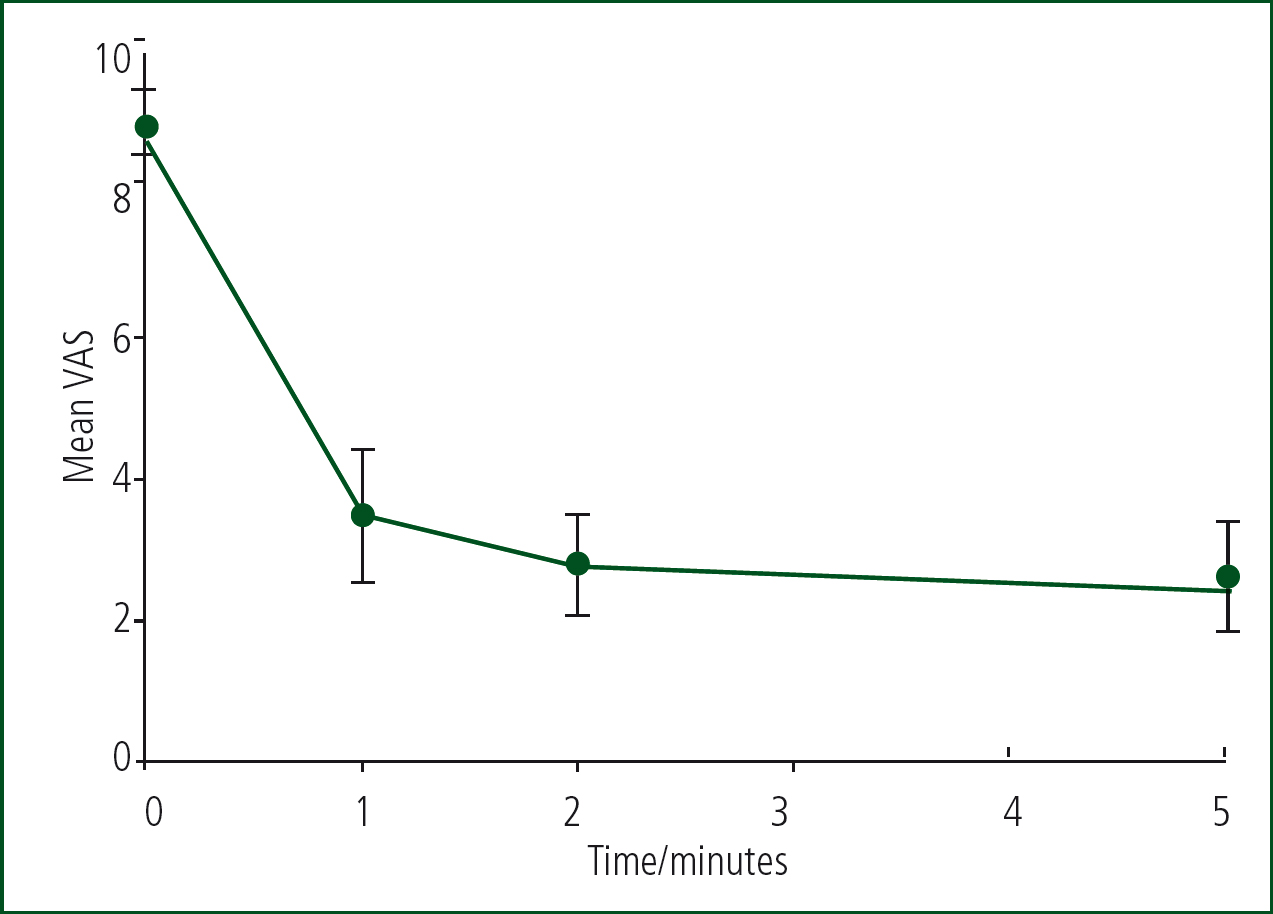
The mean VAS pain scores (Figure 2) include both polytrauma and single-system trauma. The data show a significant level of analgesia from the first time-point of 1 minute which increases up to the 5 minute time point.
There were no reported complications associated with methoxyflurane use. In 60% of cases (11 patients), methoxyflurane alone provided sufficient analgesia during the prehospital phase of patient management and no additional pain control was required.
Statistical analysis
Each time-point sample of VAS measurement was tested for departure from normality for both raw and transformed data. None of these samples reached significance on Shapiro-Wilk testing so normality was assumed. One patient had a missing VAS score at 5 minutes because they had been intubated; this patient's data were dropped from the modelling.
At an early stage in model exploration, it was established that interaction and the presence or absence of polytrauma did not provide a significant contribution to the explanatory power of the model and this was dropped from the final model. The polytrauma and single injury scores were therefore combined for further analysis. Correction for autocorrelation did not improve the model and was also dropped.
Repeating the process with a square-root transformed response variable improved the model significantly. Therefore, the minimal adequate model was identified as one with square-root transformed VAS as the response variable, time of VAS assessment as the only fixed effect, the individual patient as a random effect and no correction for autocorrelation. The residuals of the final model were checked for normality with a quantile-quantile plot and Shapiro-Wilk testing. No significant deviation from normality was detected.
Compared with before administration, there was a highly significant fall in VAS pain score (P<0.0001) at all time points. Effect sizes (calculated after reversing the transformation) were reduction in pain score of 5.5 at 1 minute, 6.2 at 2 minutes and 6.6 at 5 minutes from an average of 8.8 before administration.
Discussion
The mean VAS patient pain scores at 1 minute, 2 minutes and 5 minutes after administration of methoxyflurane demonstrate its efficacy of as early as 1 minute after administration, with a reduction of more than 5 points in the VAS pain score (Figure 2). The VAS scores continued to decline over the 5-minute period, with most patients moving from severe pain (VAS score ≥8) to mild pain (VAS score ≤3) within 5 minutes of methoxyflurane administration (Figure 2). This is a significant and rapid onset of effective analgesia.
The speed at which analgesia was obtained enabled a shorter time to patient assessment and initiation of care as many people cannot be fully assessed and/or managed because of their level of pain.
Methoxyflurane can be used at the first point of patient contact, either as definitive pain relief or as a bridge until the patient is successfully cannulated and intravenous analgesics administered; for example, it could be administered to an elderly patient with a neck of femur fracture in extreme pain and no visible veins for vascular access.
As a self-administered inhalatory analgesic, the attraction here is its rapid onset, usually over 6–10 breaths (Coffey et al, 2014) and the fact that it does not have a significant effect on blood pressure (Oxer, 2016), respiratory rate (Oxer, 2016) or conscious levels. The 3 ml inhaler will last for 25–30 minutes if used continuously or up to an hour with intermittent use and a second 3 ml dose can be administered (maximum permitted dose is 6 ml in any 24 hours) (eMC, 2018).
Compared to nitrous oxide/oxygen (Entonox), as an example, Penthrox can be used over a wide range of environmental temperatures and the device is light in weight, easy-to-use and portable.
Opiates require cannulation, which may be a challenge early in the prehospital environment, and their use is restricted. Methoxyflurane can be provided through a patient group directive (PGD).
With increasing use (some on trial) of methoxyflurane by ambulance services, helicopter services and British Association For Immediate Care (BASICS) schemes as well as EDs, there is scope for more detailed evaluations.
Limitations
This is a simple observational study undertaken by two individual practitioners. Despite the extensive use of methoxyflurane in Australasia, there is little research comparing it with other analgesics. In an RCT, Lim et al (2016) compared it to IM tramadol (100 mg), which showed methoxyflurane resulted in a significantly greater reduction in patient pain scores, with a faster time to onset of pain relief.
Its effectiveness has been examined in the STOP study (Coffey et al, 2014), a randomised double-blind, placebo-controlled study, which demonstrated its efficacy and safety.
Conclusions
Methoxyflurane (Penthrox) is a self-administered inhalatory analgesic. While it is new to the UK, it has a proven safety record and a history of more than 5 million uses in Australia.
With the drug being increasingly used in prehospital care and EDs, it is becoming more recognised as a useful adjunct to the choice of analgesia for use in trauma patients with moderate-to-severe pain. This case series shows it produces statistically significant improvements in patient pain scores at 1, 2 and 5 minutes.

