Between 1 April 2017 and 31 March 2018, ambulance services attempted to resuscitate 32 099 people in England alone (NHS England, 2018), achieving a return of spontaneous circulation (ROSC) in 9846 (30%) patients that was sustained to hospital. Work is continuously being undertaken to improve out-of-hospital cardiac arrest (OHCA) survival. Patients who have experienced an OHCA with subsequent ROSC can present with a complex array of symptoms, and are at particular risk of respiratory and cardiovascular compromise.
The optimal prehospital care strategies for patients experiencing an OHCA are not yet fully understood (Adrie et al, 2004; Neumar et al, 2008). Of patients who receive advanced airway management during resuscitation, only a minority will regain sufficient consciousness in the prehospital phase to allow step down to basic airway management. With an increasing emphasis on primary transfer of OHCA patients to regional specialist centres, both urban and rural paramedics could find themselves in a position where they are unable to ventilate or oxygenate their patient effectively, or prevent aspiration and potentially lose airway patency in a patient still highly reliant on ventilatory support or airway control.
Resuscitation Council (UK) (2015) guidelines recognise the likelihood of agitation or combativeness after ROSC, and suggest giving incremental doses of benzodiazepines (such as diazepam or midazolam). This could potentially be an effective firstline response, but it is not endorsed in the national clinical guidelines of the Joint Royal Colleges Ambulance Liaison Committee (JRCALC) (Association of Ambulance Chief Executives (AACE), 2019). Furthermore, hypotension is a common side effect of benzodiazepines (Joint Formulary Committee, 2019). It is quite feasible that some post-ROSC patients may be too cardiovascularly unstable to tolerate deep sedation using benzodiazepines, yet may exhibit agitation and intolerance of an airway control or ventilation strategy with light sedation.
In countries where paramedic-delivered rapid sequence induction of anaesthesia (such as Canada and the United States) is used, neuromuscular blocking agents (NMBAs) are administered alongside sedatives and analgesia (West Virginia Office of Emergency Medical Services, 2009; Rhode Island Department of Health, 2017) in patients experiencing ROSC after OHCA. The UK, however, has traditionally had no such facility, and there is no option to sedate a post-ROSC OHCA patient in standard UK paramedic practice.
To meet the needs of this highly dependent group of patients, Southeast Coast Ambulance Service NHS Foundation Trust (SECAmb) has a group of specialist paramedics—critical care paramedics (CCPs). CCPs are experienced paramedics who have completed a postregistration course at master's level, and who meet every 7 weeks for further governance, training and peer review of incidents. CCPs are tasked selectively to high-acuity incidents, and have an increased scope of practice including interventions such as inotropic infusions, sedation, pacing and cardioversion, as well as surgical crichothyroidotomy as an emergency airway rescue technique. Among the post-ROSC care package that a CCP can tailor to the patient, SECAmb has offered rocuronium bromide since 1 April 2016.
Rocuronium is a non-depolarising neuromuscular blocking agent (NMBA), and has a recognised place in the care of patients who have achieved ROSC and require mechanical ventilation to control hypoxaemia and hypercapnia, and to reduce patient-ventilator dysynchrony (Nolan et al, 2015). All interventions carry risks as well as benefits, and in the case of NMBAs, these risks can be significant if not robustly managed and mitigated.
The Association of Anaesthetists of Great Britain and Ireland offers guidance on risk management in neuromuscular blockade (Lockey et al, 2017), and this report is one part of SECAmb's governance processes underpinning NMBA use. Figure 1 shows the general process and requisites within which a CCP could give rocuronium in this report.
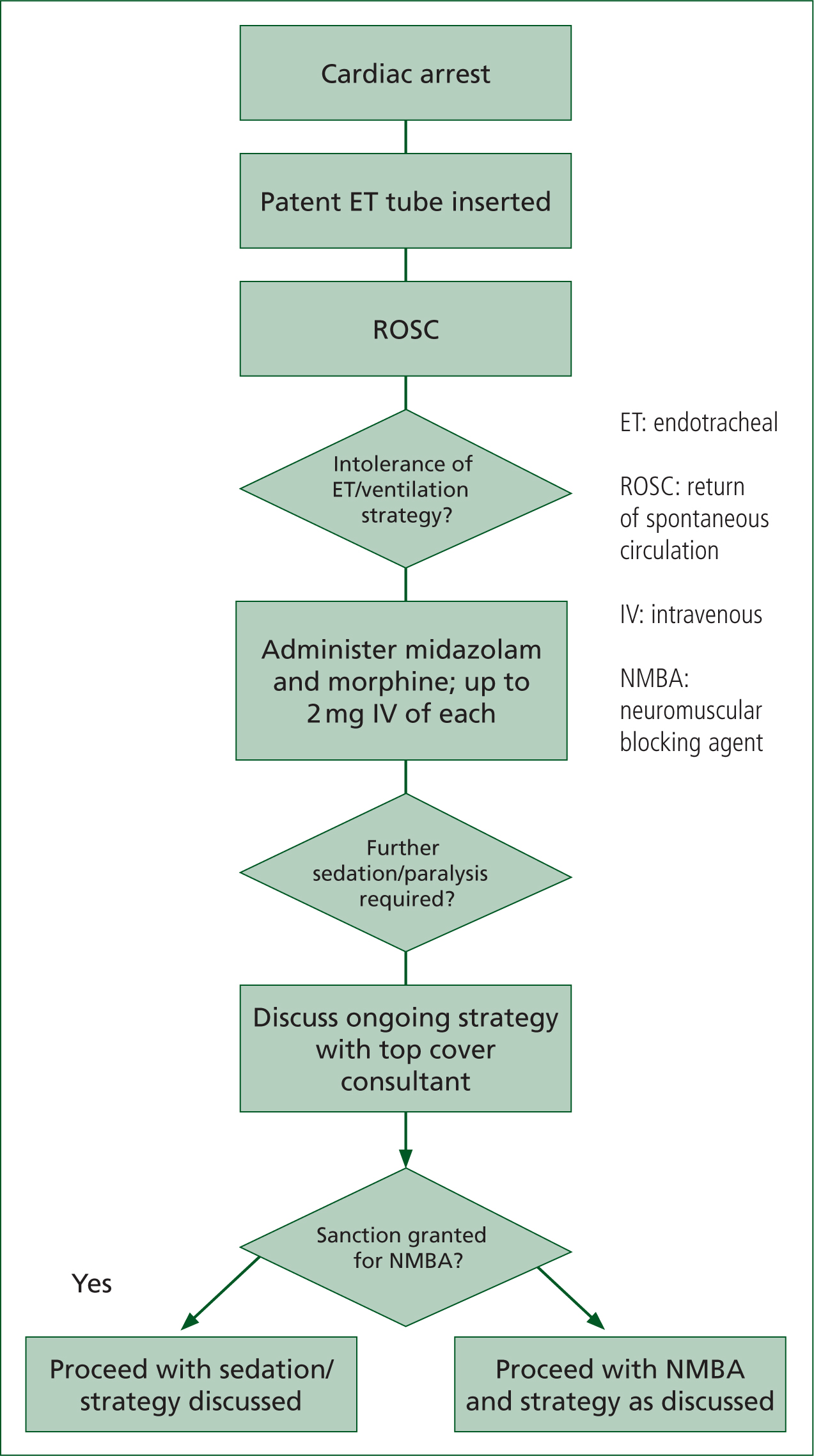
SECAmb is the first and, at time of writing, believed to be the only NHS ambulance service to be providing non-physician delivered neuromuscular blockade in this way, which makes providing any national comparator almost impossible.
Aim
The aim of this study was to report on the administration of paramedic-delivered NMBA, which started on 1 April 2016 at SECAmb, and examine this practice innovation and its acceptability as an effective prehospital intervention.
Methods
Design
This was a retrospective service evaluation using routinely collected data from National Reporting and Learning System (NRLS) submissions, dispatch system records and practice governance databases (described in more detail under the ‘Data collection’ section of the current article).
Setting
SECAmb is an NHS foundation trust ambulance service that covers urban, suburban and rural areas in the south-east of England. At present, local agreements with receiving hospitals and specialist centres are inconsistent in terms of the initial destination of post-ROSC patients. Annual calls number around 187 000, of which just over 8000 are cardiac arrests (including those where no resuscitation attempt is made). During the study period, CCPs attended around 5500 cardiac arrests.
Participants
All patients who received rocuronium within SECAmb and whose airway was controlled using an endotracheal (ET) tube were included. By definition, these were patients who had experienced an OHCA, achieved an ROSC, then began to display intolerance of their airway or ventilation strategy. Patients were excluded if their airway was managed using a supraglottic device.
Data collection
All administrations of rocuronium and the circumstances surrounding them are routinely self-reported by the CCPs for audit and review purposes.
The database on which these data are recorded changed during the data collection phase of this study. Between 1 April 2016 and 1 February 2017, data were recorded on the advanced lifesaving intervention procedures (ALSIP) database—a standardised reporting sheet using Microsoft Word. Since 1 March 2017, they have been recorded on a purpose-designed electronic database called CCPBase (Medic One Systems). The data stored in both of these databases are largely the same: a record of the clinical picture surrounding any advanced interventions, as well as the decision-making processes underpinning them. The newer CCPBase system can store uploads from the patient-monitoring machine used.
Clinical and operational notes, comprising handwritten patient report forms, dispatch notes, LifePak 15 monitor uploads (Physio Control) via LifeNet systems, and the CCP's own report to CCPBase/ALSIP database were hand-searched by the primary author.
Operational notes were accessed via the SECAmb computer-aided dispatch (CAD) system records. In all cases, the programme's quality improvement lead checked data abstraction.
Outcome measures
Adverse incidents were defined by the consultant team and classified into seven categories: administration errors (circumstances when the drug was administered did not fall within the wording of the patient group direction (PGD); anaphylaxis; extubation; airway issues (including hypoventilation from any other cause); re-arrest (attributable to rocuronium use, rather than clinical/pathological course); phlebitis; and handover issues.
The patient's destination, as well as the time period between rocuronium administration and arrival at hospital (i.e. when anaesthetic support was first available) was taken from patient records, CCPBase, and the trust's CAD records.
Survival-to-discharge data were retrieved from the routine NRLS submissions.
Statistical analysis
All data were collated and processed on a spreadsheet using Microsoft Excel. Descriptive statistics were used to describe the cases, including patient demographics, prehospital diagnosis, time to hospital, destination and survival to discharge, as well as the occurrence of any adverse event.
Ethical approval
Using standard decision-making software from the NHS Research Authority (2017), this study can be classed as a service evaluation. All data used to compile the report were collected routinely as part of standard governance processes. Ethical approval was therefore not required.
Results
The first administration of rocuronium by a UK paramedic was on 17 April 2016 and data were collected from all cases (n=127) up to 22 July 2017. Baseline characteristics of the patients are shown in Table 1.
| Age (years), mean ± SD | 63 ±18 | |
| Sex, n(%) | Male | 89 (70%) |
| Female | 38 (30%) | |
| Weight in kg, mean (SD) | 80 (19) | |
| Arrest aetiology (working diagnosis or confirmed) n (%) | MI | 52 (41%) |
| Unknown | 44 (35%) | |
| Hypoxia | 10 (8%) | |
| Seizures | 6 (5%) | |
| Drug toxicity | 4 (3%) | |
| Cardiac problems (other than MI) | 3 (2%) | |
| Electrolyte imbalance | 3 (2%) | |
| Bleeding (medical cause) | 3 (2%) | |
| Time from NMBA administration to arrival at hospital, median (IQR) | Trauma | 2 (1%) |
| 32 minutes | ||
| (20–43 minutes) |
MI: myocardial infaction; NMBA: neuromuscular blocking agent; IQR: interquartile range
Time to hospital
Accurate data were available for both time of NMBA administration and time of arriving at hospital in 88 cases (69%). The amount of time between the patient receiving NMBA and hospital arrival varied widely. Median time was 32 minutes, and the interquartile range (IQR) was 20–43 minutes. Further doses (given based on patient need after 45 vminutes) were given in 27 cases.
Destination data were available for 112 patients (88% of the total) (Table 2). The patient whose destination was unknown was conveyed by the local helicopter emergency medical service (HEMS) team.
| Destination | n (%) |
|---|---|
| Emergency department (ED) | 80 (71%) |
| Catheterisation suite direct | 22 (20%) |
| Catheterisation suite via ED | 9 (8%) |
| Unknown | 1 (1%) |
Survival to discharge
Outcome data were available for 89% of the patients (n=119). Of these, 31% survived to discharge (n=37).
Adverse incidents
Over the study period, there were six instances (4.7%) that could be initially categorised for the purposes of this study as an adverse incident. Each incident was reviewed by a member of the consultant team (who had not been the authorising consultant). On review, three of these were reclassified as non-adverse; in each case, this was because the incident was a ‘re-arrest’ and, after a review of the patient notes and monitoring records, these were deemed to have been pre-existing pathological course, rather than resulting from rocuronium administration. After review, therefore, three patients (2.3%) were found to have experienced an adverse incident (Table 3).
| Frequency (%) | |
|---|---|
| Handover issues | 2 (1.6%) |
| Administration errors | 1 (0.8%) |
| Anaphylaxis | 0 |
| Extubation | 0 |
| Phlebitis | 0 |
| Rearresty* | 0 |
The two instances of handover incidents were different, but both minor. In one case, the patient was palliated on arrival at hospital but the receiving hospital said they were unable to withdraw care until the NMBA had worn off. In the other instance, triage to percutaneous coronary intervention was delayed while the receiving hospital organised an anaesthetist to help receive the patient.
The administration error incident concerned clinical management plan (CMP)/PGD non-compliance. NMBA was given on the request of an anaesthetist on scene who was not part of the official on-call team (attempts had been made to contact the on-call team). Retrospective review of the clinical picture has suggested that the patient was appropriate to receive NMBA. There were no instances of extubations, nor major airway complications.
Discussion
Among the 127 patients reported on in this study, there were three adverse incidents, one regarding CMP non-compliance and two related to the integration between the hospital and prehospital phases. After NMBA administration, these patients spent a median 32 minutes (IQR 20–43 minutes) before arrival at hospital; this was the emergency department in the majority of cases (71%; n=80) but a significant number (28%; n=31) were taken directly to a cardiac centre. With no major airway complications in this dataset, the authors feel that the findings from these data suggest paramedic administration of neuromuscular blockade in this way is at least as safe as standard paramedic practice. Standard paramedic practice here would involve the removal of airway protection should a patient become intolerant of it.
Looking at the adverse incidents from this report's dataset, the categories they fall into are of interest. It is reassuring that there were no major adverse incidents involving physiological compromise (anaphylaxis, extubation, phlebitis or re-arrest) attributable to the use of rocuronium in these patients. Adverse incidents from CMP/PGD non-compliance cannot be tolerated, and these incidences have already been addressed internally.
The two minor incidents arising from handover issues highlight the importance of liaising with receiving hospitals about what they can expect from patients they are about to receive. This is essential to allow them to more effectively cater to these patients' complex needs, and to provide a seamless patient journey from one phase of care to the next.
Since standard paramedic practice has no contingency for a patient becoming intolerant of their airway protection because of a gag reflex or fighting their ventilation strategy, 32 minutes (median; IQR 20–43 minutes) was observed in the context of these patients as the amount of time they might otherwise have been without a definitive airway because it had to be removed or without optimal ventilation because they could not tolerate it. The wide IQR is most likely driven by two factors in particular:
If these two things did contribute to some of the longer delays, it seems likely that the increasing emphasis on regionalisation of specialist care (Suntharalingham et al, 2014; Batchelor et al, 2017), and the increasing levels of obesity among the public (NHS Digital, 2018) might mean that patients may in the future be spending more time reliant upon paramedics for their ventilation and airway support while they are being extricated and transferred to a specialist facility.
Paramedics in other countries, such as the United States, have been routinely administering rocuronium for some time (Roantree and Goldstein, 2018). Frustratingly, however, this is in large part embedded within protocols for rapid sequence induction of anaesthesia (West Virginia Office of Emergency Medical Services, 2009; Rhode Island Department of Health, 2017). The authors were unable to find any studies looking at rocuronium use (administered by paramedic or physician) to facilitate airway control and ventilation in patients who were intubated during a medical cardiac arrest. Therefore, while numbers for survival-to-discharge may seem promising, no direct comparison can be made with other data at this point. However, some papers could allow an indirect comparison to be made with great caution to provide some context and perspective.
Miller et al (2017) offer useful, if perhaps unsurprising, evidence on haemodynamic changes that medical ROSC patients undergoing a similar drug regimen might display. This study was an evaluation of ROSC patients attended by HEMS teams after OHCA, who received rapid sequence induction of anaesthesia for similar reasons to those described above. Specifically, they describe hypotensive episodes in up to 16% (n=10) patients following a regimen of midazolam, fentanyl and rocuronium. Interestingly, three of their patients (5%) showed a rise in blood pressure. It is unclear from the data whether this was a pain response, but this should be borne in mind given that the analgesic was a stronger opioid than the one used in Miller et al's (2017) study, and the hypnotic agent slightly stronger (median initial dose of midazolam given was 0.04 (0.03–0.05 [0.01–0.10]) mg/kg, whereas in the present study, CCPs could administer aliquots of up to 2 mg before further authorisation was needed. In a 75 kg patient, this would be 0.027 mg/kg.
Strengths and limitations
Because of this study's retrospective nature, the authors had to assume that adverse events had been reported accurately and completely. Some may not have been reported and subsequently would not have been included in this report.
As well as this, with no direct comparators and previous work to build upon, it is unclear whether the definitions of adverse events were exhaustive, or whether there are types of adverse events that exist in the prehospital arena which have not been examined.
A further limitation is the relatively small cohort size; larger numbers will always offer greater power to draw stronger conclusions.
That said, the authors believe that a significant strength of this study is that this is the first to report widely on paramedic-administered NMBA for intubated post-ROSC patients in the UK. It is hoped that this initial groundwork can be informative and helpful in the future development and governance of NMBA use in this patient group, and can itself provide a comparator for future studies.
Future research
A large randomised controlled trial would be needed to determine whether paramedic-administered rocuronium in intubated patients who have experienced ROSC improves outcomes.
A standardised reporting system should be developed so that ongoing audit and future research can be compared and meta-analysed.
Adverse incidents should also be defined according to categories that are aligned with other published material, again to allow future comparison in audit.
Conclusion
Patients experiencing ROSC can be uniquely complex and highly dependent. With recent improvements in resuscitation and ROSC rates, not to mention mounting emphasis on increasing transport times in favour of direct transport to specialist care, safe and effective airway-control strategies for these patients are increasingly needed.
Since 1 April 2016, SECAmb has offered rocuronium bromide as an NMBA to assist with ventilator synchrony. This service evaluation set out to assess the safety of this intervention. Paramedic-administered rocuronium in intubated post-ROSC patients showing airway intolerance or ventilator dysynchrony appears safe; however, further prospective research is required to determine whether this improves patient outcomes.

