The prehospital landscape is unrecognisable from how it was 30 years ago and could be poised for a dramatic shift towards rapid diagnostic capabilities for paramedics, with point-of-care (PoC) biomarker testing.
The challenge for paramedic practice is its heavy reliance upon prehospital electrocardiography and patient symptomology to diagnose acute myocardial infarction (AMI), which may delay treatment and definitive care (Chacko et al, 2018). PoC cardiac biomarkers could be the missing link to resolve diagnostic uncertainties of AMI and subsequent decision-making in paramedic practice (Pedersen et al, 2019).
This article explores and discusses the value of introducing PoC dual-marker testing (cardiac troponin-T (cTnT) and copeptin) as an adjunct to aid diagnosis and decision-making for treatment and triage of AMI in UK paramedic practice. The cost-effectiveness, feasibility and the future of this technology will also be discussed.
Methodology
Preliminary searching of the extent of the literature determined that the body of evidence surrounding cardiac biomarkers in the prehospital setting was limited. Because the literature was so sparse, a generalised exploration of the literature was performed to evaluate the feasibility and potential application of cardiac biomarkers in the prehospital setting.
Electronic databases including the MAG Online Library, PubMed, the Cochrane Library and Embase were searched for eligible articles, ranging from 2010 until the present to maximise the available evidence base. Homogenous search criteria were used for all databases with search terms determined from medical subject headings (MeSH) terms through PubMed (Box 1) (Chapman, 2009). Articles were excluded if the full text was unavailable, not in the English language or did not refer to cardiac biomarkers such as cardiac troponin-T or copeptin in the prehospital setting.
Gap in prehospital cardiac care
‘Time is myocardium’ is the guiding principle in AMI and the earlier the diagnosis, treatment and triage provided by paramedics, the better the patient's outcome (Gibson, 2001: 2634; Faramand et al, 2019). Common UK prehospital practice is that paramedics provide prehospital thrombolysis or bypass the emergency department (ED) to cardiac catheterisation centres for coronary angioplasty and reperfusion when patients present with clear ST-segment elevation myocardial infarction (STEMI) (National Institute for Health and Care Excellence, 2013; Marcolino and Ribeiro, 2019).
However, when patients present to paramedics with non-conclusive electrocardiograms (ECGs) or atypical symptomology, they are transferred to the ED for cardiac biomarker testing and may experience diagnostic delays and delayed reperfusion (IJkema et al, 2014). This is a substantial concern, considering that a UK-wide audit suggests that more than two-thirds of all AMIs present as non-conclusive or non-ST segment elevation myocardial infarction (NSTEMI) on ECGs (Myocardial Ischaemia National Audit Project, 2017). This is compounded by a growing elderly population, who are more likely to experience NSTEMIs because of occlusive coronary artery disease and anastomotic collateral arterial flow (Chen et al, 2017).
Atypical symptomology further complicates AMI diagnosis. A high-quality paper with good effect estimation (study power >85%) and robust population validity (n=434 877) stated that only 33% of AMIs presented with chest pain in the ED (Canto et al, 2000). Certain patient groups—people with diabetes or renal disease, elderly people and women—have vague presentations such as dyspnoea and diaphoresis, creating additional diagnostic ambiguity in suspected AMI (Body et al, 2010; Ahmed et al, 2018). Therefore, an awareness that patients may not present with classic textbook symptomology creates difficulties for AMI diagnosis by paramedics (Chacko et al, 2018).
Ultimately, this uncertainty results in diagnostic delays in the ED, without definitive reperfusion and with irreparable damage to the myocardium (Terkelsen et al, 2010).
This is supported by a high-quality, 15-year longitudinal study that showed patients conveyed to the ED with an NSTEMI experienced delayed angioplasty (compared to direct access coronary intervention), increased risk of congestive heart failure and cardiac arrest (Arora et al, 2018). This highlights the need for early and rapid diagnostic capabilities of AMI by paramedics, which could be accomplished by PoC cardiac biomarkers (Freynhofer et al, 2012).
Point-of-care cardiac biomarkers
Cardiac biomarkers that are elevated in the serum are surrogate markers for myocardial ischaemia and necrosis; they provide the gold-standard diagnosis of AMI (Bingisser et al, 2012). Traditionally, cardiac biomarkers are measured in the laboratory with venous samples (Chapman et al, 2019). However, recent advances allow rapid measurements from capillary blood samples with PoC handheld devices that could be used in the prehospital environment.
The most well-established PoC cardiac biomarker is cTnT, but copeptin has recently gained precedence (Bingisser et al, 2012). However, all biomarkers differ in sensitivity and specificity, diagnostic value and kinetics (Nagesh and Roy, 2010).
CTnT is a cardiac biomarker released after disruption to the cardiomyocyte sarcolemmal membrane following myocardial ischaemia or necrosis, with a consensus suggesting high specificity (97% (96–98%)) (Reichlin et al, 2009; Xu et al, 2013). Serum cTnT increase is correlated linearly with the presence and size of an AMI, as well as guiding optimum treatment (thrombolytics and/or primary angioplasty) (Gimenez et al, 2017). However, cTnT is detected in serum only from 3–12 hours after infarct, creating a ‘troponin-blind’ period (Figure 1), which can reduce its sensitivity (72% (64-80%)) (Reichlin et al, 2009; Wu, 2017). This is problematic for paramedics, as PoC cTnT may be absent in samples in the early stages of AMI development (Suzuki et al, 2018). Therefore, another marker released immediately from AMI onset should be considered—copeptin.
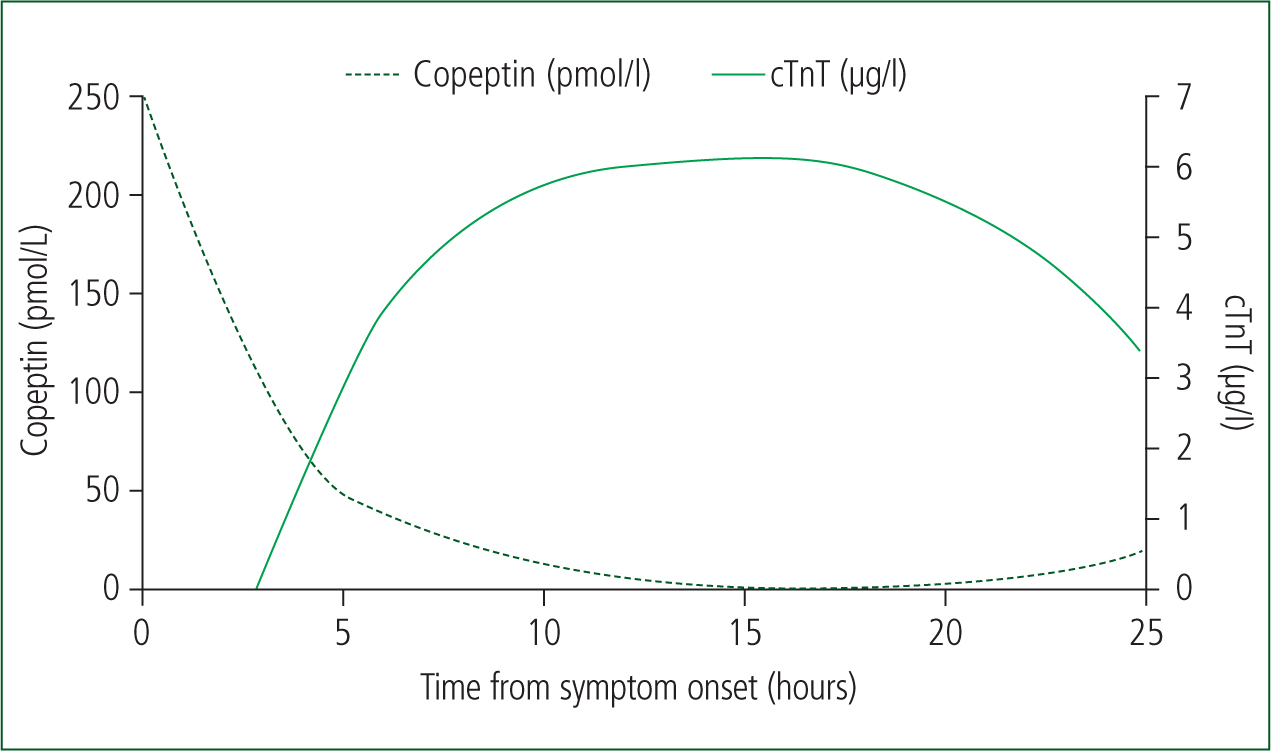
Copeptin is an endogenous stress neuropeptide, with an immediate concentration peak (0–4 hours) from AMI onset, without the problematic troponin-blind period (Figure 1) (Nagesh and Roy, 2010; Lipinski et al, 2014). The consensus of evidence (high sensitivities 98.8% (85.1–99%)) of copeptin demonstrates its key strength over cTnT, as it is the common time frame of paramedic encounters for this patient cohort (Keller et al, 2010; Nickel et al, 2012).
High-quality literature has confirmed that increased serum copeptin is correlated with myocardial necrosis and subsequent left ventricular failure so can be a proxy for the presence of an AMI (Reinstadler et al, 2015). However, in isolation, it can create diagnostic confusion, as a result of a consensus that suggests lower cardiac specificity (47.3% (34.9–53.5%)), as it is released in other conditions such as sepsis, heart failure and respiratory infections (Lotze et al, 2011; Morawiec et al, 2018).
To counteract the limitations of both singular biomarkers (cardiac specificity and time frame), the dual-marker strategy of PoC cTnT and copeptin has recently gained attention (Raskovalova et al, 2014). One robust meta-analysis demonstrated the dual-marker strategy covered all time frames after the onset of AMI, permitting improved and rapid diagnosis (Lipinski et al, 2014).
Paramedics could use this tool for risk stratification and as an adjunct in clinical decision-making for the likelihood of an AMI in the context of each patient. This would enable triage to definitive care (invasive cardiac centres) or start early treatment (prehospital thrombolysis) before attending the ED (Alghamdi et al, 2019).
Why use the PoC dual-marker strategy?
The paramedic profession is evolving by triaging patients to specialist care such as major trauma centres, hyperacute stroke units and invasive cardiac centres (Austin et al, 2019). Despite such developments, patients are less likely to be accepted by invasive centres with atypical AMI presentations or non-conclusive ECG changes, resulting in late or missed diagnoses (Docherty et al, 2018). Greater diagnostic tools such as dual-marker PoC testing would use frontline paramedics efficiently to rapidly diagnose AMI and redirect patients along the care pathway to the right treatment (Rasmussen et al, 2019).
For the best diagnostic outcome, both cardiac biomarkers should be measured as early as possible, particularly considering the temporal release of copeptin (Figure 1) and the high mortality rate of AMI (Stengaard et al, 2017).
One cohort study illustrated a significant early rise in copeptin and low levels of cTnT in prehospital PoC measurements, but an absence of copeptin in ED measurements (Slagman et al, 2015). This study highlights the time-sensitivity of copeptin, which may be present only in prehospital sampling. Comparable conclusions were drawn in a systematic review with robust research methodology by Li et al (2014), further strengthening the benefits of paramedics using the PoC dual-marker strategy for AMI diagnosis.
All studies exemplify that paramedics are in the best position to measure these time-sensitive biomarkers, being first responders to AMIs (Nagesh and Roy, 2010).
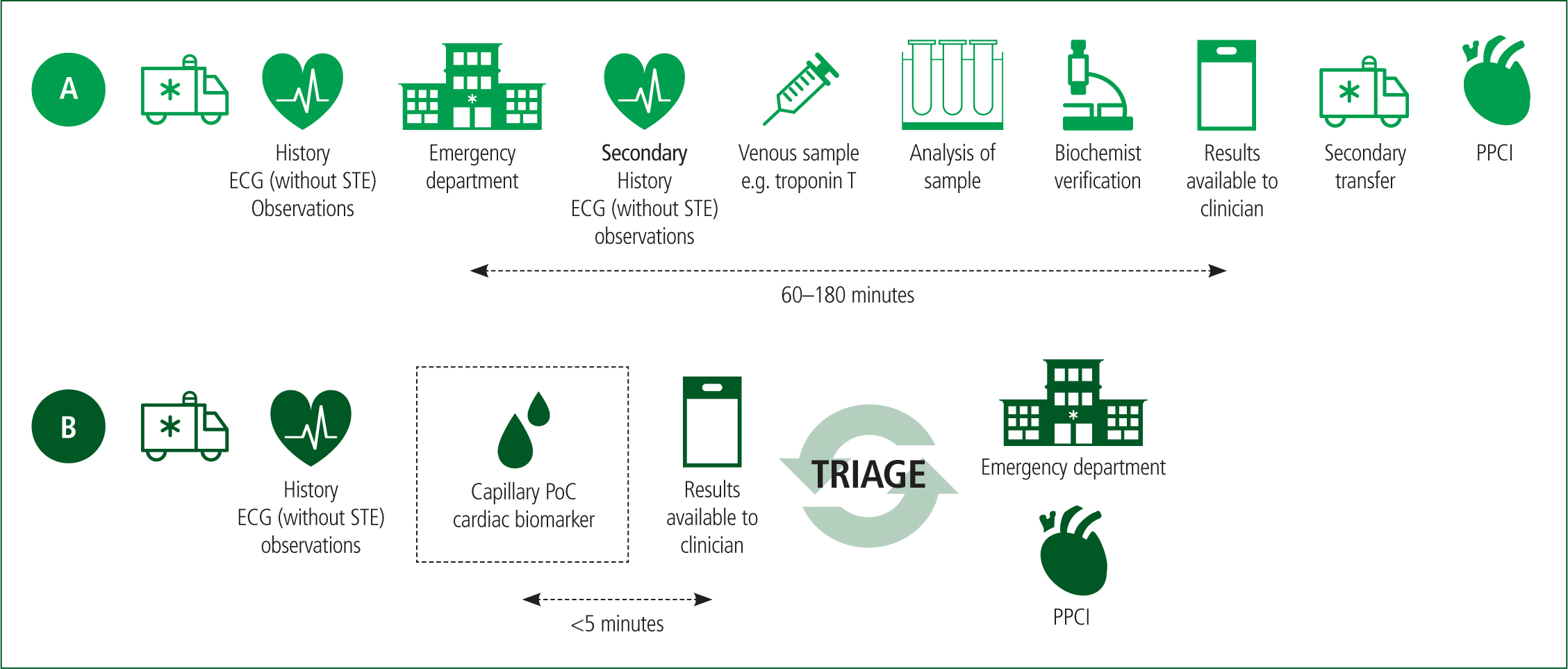
Another key advantage of prehospital PoC biomarker testing is the rapid analytical turnaround times (0–10 minutes), compared to central laboratory measurements (60–180 minutes), according to a systematic review by Bingisser et al (2012). Although this study was conducted nearly 10 years ago, overall, it is high-quality literature with study homogeneity, and recent meta-analyses support its conclusions, which suggests they are valid (Arslan et al, 2019; McCoy et al, 2019).
Therefore, paramedics could use PoC testing technology to risk stratify patients earlier, reducing delays to definitive care by field triaging suitable candidates to invasive cardiac centres or treating them with thrombolytics (Rasmussen et al, 2019). Considering UK ambulance service demand and delayed attendances, this would surely benefit patient care as it could prevent further delays to treatment resulting from conveyance to an inappropriate facility and secondary transfers being required (Figure 2) (Slagman et al, 2015).
Dual-marker PoC testing has a similar diagnostic and analytical performance to laboratory measurements (Juliano and Wason, 2017). One cohort study found no statistically significant differences in sensitivity and specificity between PoC and laboratory measurements, which were capable of detecting both biomarkers above the 99th percentile of the upper reference limit to suspect AMI (Stengaard et al, 2017). However, the inclusion criteria at the discretion of the attending paramedic may introduce selection bias, as it is subject to individual clinical education, experience and knowledge. This could be problematic as external validity to healthcare may be limited without objectivity of the attending clinician (Nour and Plourde, 2019).
Despite this, there were comparable observations in a robust meta-analysis, suggesting valid conclusions (Shin et al, 2018). This further supports that a PoC dual-marker strategy can provide paramedics with handheld devices with laboratory-quality diagnostic capabilities and the confidence to suspect AMI. This would enable attending clinicians to start early treatment (thrombolysis) or redirect suitable candidates to cardiac catheterisation centres, reducing diagnostic delays (Alghamdi et al, 2019).
Earlier AMI diagnosis would surely benefit patients by reducing mortality and morbidity from earlier treatment and earlier reperfusion (Roffi et al, 2016). This is imperative, considering that the greatest myocardial salvage occurs within the 2–3 hours from onset of symptoms (Gershlick et al, 2013).
Training needs for PoC cardiac biomarkers
Limited literature explores the specific training required for PoC dual-marker testing but the principles parallel capillary blood glucose testing, performed in daily paramedic practice (Loewenstein et al, 2013). However, clinical acumen combined with an understanding of the pathophysiology of AMI, biomarker kinetics and cut-off values would be required (Nagesh and Roy, 2010).
Initial implementation could use a protocol-based system, where patients presenting with AMI symptomology could have capillary blood samples taken and cTnT and copeptin measured by a PoC device. Values above a cut-off value could provide further evidence of an ongoing ischaemic event, and the possibility of an AMI (Pedersen et al, 2018). This would ensure a simple integration into practice and, with increased confidence, paramedics could eventually develop greater autonomy in the use of this technology (Pedersen et al, 2018).
Nevertheless, further research is required to determine the specific training for each ambulance service trust before cardiac biomarker PoC testing can be fully implemented into paramedic practice.
Cost, feasibility and future of PoC testing for cardiac biomarkers
Commonly cited barriers to the implementation of PoC technology include the cost as well as prehospital dynamics (temperature, vibrations and deceleration and acceleration forces), which may influence its function (Dahlgren et al, 1997; Chapman et al, 2019).
Devices such as the Abbott i-STAT 1 could have potential as it measures troponin I as well as carry out biochemical and haematological analysis (Abbott, 2022). Although it does not measure copeptin and cTnT currently, it does demonstrate that there are devices that can measure biomarkers in the prehospital setting. This unit costs £2000 but this amount could be easily recovered by the NHS in the long term from earlier triage and reperfusion, reduced length of hospital stays and fewer secondary ambulance transfers (Rasmussen et al, 2019; Abbott, 2022). Earlier reperfusion may also reduce complications such as chronic heart failure and the associated financial burden for its long-term management (Paradies et al, 2018).
The prehospital environment is a complex, dynamic environment, subject to temperature variations, acceleration and deceleration forces during emergency driving, and associated vibrations from road surfaces compared to the regulated, sheltered hospital environment (Dahlgren et al, 1997). This may limit PoC testing in some circumstances, which should be taken into account when considering the future feasibility of this technology.
Future research may lead to the discovery of cheaper alternatives or robust devices (that can deal with temperature fluctuations and vibrations) like the capillary microfluidic chip, which could easily be implemented into paramedic practice, being compatible with iPads carried by frontline paramedics in UK trusts (Liang et al, 2019). Nevertheless, the diverse capabilities (measuring blood gases and electrolytes) of this device could fit the demands of the modern paramedic for safer referrals and discharges and alleviate the demands upon acute care hospitals (Knowles et al, 2020).
Few UK ambulance services are trialling PoC cardiac biomarkers, but the PRe-hospital Evaluation of Sensitive TrOponin (PRESTO) trial may shed light on its capability in the near future (Alghamdi et al, 2019). Full integration of this technology could potentially modify AMI pathways, with biomarker measurements being factored into decision-making regarding treatment and the best clinical pathway (Figure 2).
In Denmark, ambulance services are trialling the PoC cTnT as an adjunct for triage decision-making to invasive cardiac centres, which may pave the way for feasibility in UK paramedic practice (Pedersen et al, 2018).
Research demonstrates that there may also be potential for using this combination of cardiac biomarkers in rule-out strategies but this is also in its infancy (Möckel and Searle, 2014).
Conclusion
The introduction of PoC dual-marker strategy (cTnT and copeptin) in paramedic practice could improve prehospital capabilities and assist paramedic decision making. High-quality evidence shows that this method is rapid, accurate and could improve the recognition of atypical AMI presentations in paramedic practice.
Paramedics are in the prime position to measure cardiac biomarkers and consider their release into serum. Combined with clinical acumen, this would empower clinicians to triage to invasive centres for gold-standard care.
Early prehospital diagnostic of AMI could result in earlier reperfusion, reduce complications and improve prognosis.
Subsequent research should focus on additional evidence to support the implementation of PoC cardiac biomarkers testing into daily paramedic practice, as well as the feasibility and affordability of this. Healthcare must become more efficient considering current pressures, and paramedics are an untapped source of potential for system improvements. This would benefit patient treatment and, eventually, their prognosis.