Of the 102 000 patients with sepsis who arrive annually at emergency departments in the UK, 68 000 (Smyth et al, 2016) have reportedly been conveyed by emergency medical services (EMS); 80% of them required intensive care treatment (Smyth et al, 2016), all of whom were found to have lowered vitamin C (ascorbic acid) levels (Carr et al, 2015). Recent studies in the intensive care unit (ICU) setting have identified this nutritional deficit as a key factor in the development of organ failure (Wilson, 2016).
Early clinical trials have shown that administering ascorbic acid has reduced mortality from sepsis-related organ dysfunction (Marik, 2017). However, prompt implementation seems to be a key feature for success (Boisramé-Helms et al, 2013) so it can be argued that prehospital clinicians such as paramedics are ideally placed to identify and initiate this form of treatment (Studnek et al, 2012). This is especially pertinent as those arriving by EMS are predominantly in a more advanced and critical stage of the condition (Seymour et al, 2010).
Although no studies covering prehospital trials were identified, the evidence is still overwhelmingly in support of the prompt administration of ascorbic acid as a primary therapy in the treatment of sepsis (Fowler et al, 2014) and, in light of no notable safety risks (Aschauer et al, 2014), it could potentially be used prophylactically to reduce endothelial tissue injury from systemic inflammatory responses resulting from infection (Tupchong 2015), which is a predictor of sepsis.
Objective
The objectives of this review are to: explore the potential value of intravenous ascorbic acid as a primary treatment for sepsis in the prehospital environment; and find out whether it can reduce tissue and organ dysfunction and therefore mortality rates in this patient group.
Methods
A literature search was conducted in 2018 using the Bournemouth University electronic database mySearch—an EBSCO Discovery Service platform, that gives access to open-source websites PubMed and the Cochrane Library. This process involved creating an initial search strategy which had to be adapted because of the databases’ individual functionalities. All these databases had set inclusion and exclusion criteria (Table 1); articles were limited to those written in English to avoid translational or interpretation errors (Curtis et al, 2016) and published within the previous 5 years to exclude any outdated analyses.
| Characteristics | Inclusion criteria | Exclusion criteria | Rationale |
|---|---|---|---|
| Publication language | English | Translated articles | Resources to translate the literature are limited and the author is fluent in English |
| Publication origin | Any country | No restrictions | To review the topic from a international perspective |
| Publication year | Articles published from January 2014 to April 2018 | Articles published before January 2014 | Limited by the author as newer evidence is being produced and this would be better for clinical practice |
| Publication type | Peer-reviewed journal publications | Narrative/literature reviews, letters, editorials, commentaries, books, book chapters, lectures and addresses, and consensus statements | Increased credibility |
| Types of studies | Primary research | Non-primary research such as systematic reviews, opinions and qualitative research | The aim of systematic review based on primary research |
| Methodology | Quantitative studies | Systematic reviews, opinions, qualitative research | The review question is based on quantitative studies with an outcome looking at scene time |
Keywords and their synonyms were used including sepsis, severe sepsis or septic shock, vitamin C, ascorbic acid, vitamin therapy, treatment, mortality and morbidity. Different combinations were tested to highlight all potentially relevant studies. After duplicates were removed, articles were initially screened for relevance and were included if ascorbic acid had been used as a primary therapy in the treatment of sepsis as detailed in the title or abstract. A second stage of screening involved limiting the included studies to those that measured outcomes in respect to Sequential Organ Failure Assessment (SOFA) score or biomarkers such as procalcitonin (PCT). Finally, all but human clinical trials were removed to remain within the scope of this review and its objectives.
The quality of evidence in the remaining studies was analysed and reviewed using the GRADE method (Ryan and Hill, 2016). In addition, a further model designed by Downs and Black (1998) specifically for assessing the quality of both randomised and non-randomised studies was used but adjusted for article relevance in an effort to complement the initial review. This had the benefit of offsetting any potential misnomers, assuring a thorough quality assessment had been achieved (National Collaborating Centre for Methods and Tools, 2018).
Results
The database searches yielded 28 citations. Nineteen articles were rejected during the first screening stage and a further six were discarded following review in the second stage (Figure 1). The PRISMA guidelines were followed (Moher et al, 2009).
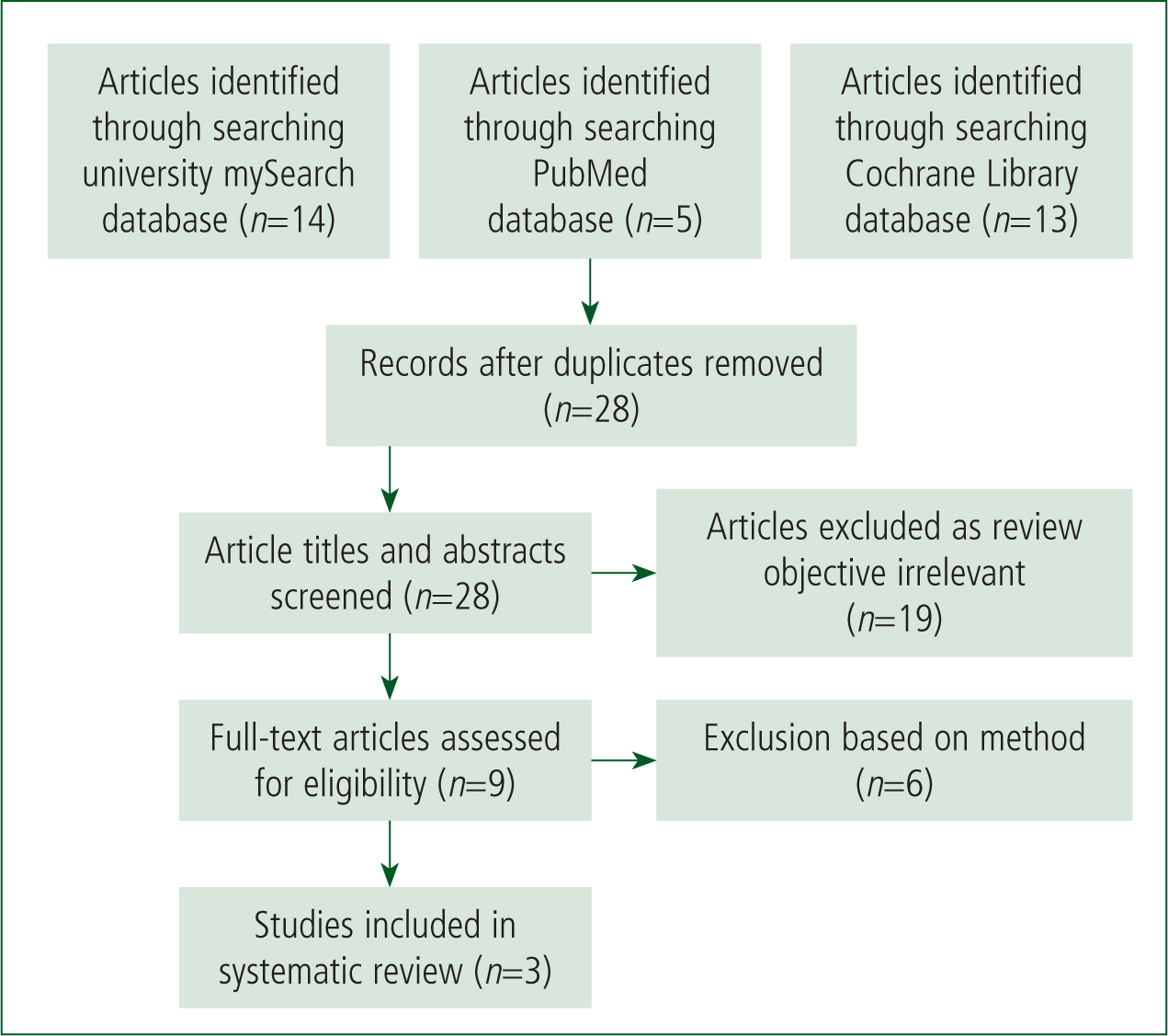
One additional study that met the inclusion criteria was identified from the reference lists of the remaining articles. However, its focus was deemed to add little given the objectives so was excluded.
This left three primary research studies for critical appraisal. One balanced placebo controlled cross-over-study conducted by Aschauer et al (2014) was deemed to be of low quality because of potential imprecision and selection bias but was included in the review as there were few comparable studies. A randomised, double-blind, placebo-controlled phase I trial by Fowler et al (2014) was considered to be of high quality because of its methodology and lack of any serious bias. Finally, a retrospective before-after clinical study by Marik et al (2017) was of moderate quality because of inconsistencies but could still reasonably be included.
The relevant data from each study were extracted by the author (Appendix 1, online). All studies were conducted in a hospital and not a prehospital environment, so both narrative and meta-analytical approaches were adopted for data synthesis within the discussion.
Discussion
Aschauer et al (2014) explored the use of ascorbic acid to restore impaired vasculature caused by endothelial dysfunction—a leading predictor of sepsis (Carr et al, 2015)—during a balanced placebo cross-over trial. They used Escherichia coli lipopolysaccharide endotoxin (LPS) to induce an acute inflammatory response within the forearms of 36 participants to measure the effects of intravenous (IV) ascorbate on forearm blood flow (FBF). The outcome was a vast improvement in peripheral perfusion (Aschauer et al, 2014).
At a later date, Fowler et al (2014) performed a randomised, double-blind, placebo-controlled clinical phase I trial to determine the safety of infusing ascorbic acid intravenously and its therapeutic effect on organ dysfunction, which is synonymous with the development of severe sepsis (Tupchong, 2015). The result of this showed a significant reduction in the proinflammatory marker PCT during therapy in all 12 treatment subjects, with an associated decrease in their SOFA score and an absence of any notable adverse effects or symptoms from the infusion (Fowler et al, 2014).
More recently, Marik et al (2017) conducted a retrospective before-after clinical study, which aimed to establish the effectiveness of vitamin C, alongside thiamine and hydrocortisone in preventing progressive organ dysfunction, a precursor to organ failure in those who experience septic shock (De Grooth et al, 2014). Once again, the results exhibited a stark improvement in all 47 patients receiving the additional therapy compared with those who received the standard, regimens (Marik et al, 2017).
According to Carr et al (2015), all septic patents have an ascorbic acid deficiency, and 88% of those admitted to an emergency department via EMS are in a state of hypovitaminosis C (a severe deficit).
Ascorbic acid is a key cellular antioxidant, which scavenges the reactive oxygen species (ROS) that are natural byproduct of cell mitochondrial oxygen metabolism (Cash et al, 2007). A deficiency in ascorbic acid can lead to an excessive build-up of these molecules, inducing oxidative stress (Teng et al, 2017), which impairs the function of epithelial cells, subsequently causing vascular tissue injury such as endothelial dysfunction (Barabutis et al, 2017). A feature of this is reduced vascular reactivity to vasoconstrictor mediators (Boisramé-Helms et al, 2013), which causes refractory vasodilation, inducing hypotension in severe sepsis and, ultimately, organ failure (Marik, 2018).
In addition, endothelial barrier dysfunction also increases the permeability of capillary walls to macromolecules (Wilson, 2013), accelerating plasma protein extravasation, which reduces the amount of circulating blood. This results in hypovolaemic shock—the critical stage in the evolution of sepsis (Seeley, 2016)—and is why endothelial dysfunction has been used as a predictor for mortality in septic patients (Aschauer et al, 2014). Herein lies one possible rationale behind the introduction of ascorbic acid as a potential therapy in those with sepsis.
Using an established model for studying endothelial dysfunction that causes a parallel reduction of plasma ascorbate, Pleiner et al (2002) measured FBF levels in 36 participants to record a baseline then inoculated them with LPS. Twelve participants were kept as a control group while two randomised treatment groups were formed, both consisting of 12 individuals who were administered either a low (160 mg/kg) or higher (240 mg/kg) IV dose of ascorbic acid to determine if it aided vascular reactivity as it had been shown to do so in animal trials (Pleiner et al, 2002). As expected, LPS impaired FBF and systemic ascorbic acid levels also decreased (Aschauer et al, 2014). Following the administration of ascorbic acid, FBF returned to 80% of baseline in both treatment groups while LPS toxins were still circulating in the blood (endotoxaemia). Those in the control group saw no return of FBF baseline over the same period.
As previously discussed, endothelial dysfunction serves as a predictor for the development of sepsis mortality, and has been shown to correlate with diminishing plasma ascorbic acid levels. While current treatment procedures focus on oxygen and fluid therapy to restore adequate perfusion, as recommended following the Surviving Sepsis Campaign (Smyth et al, 2016), these interventions fail to restore the ascorbic acid deficiency that evidently accompanies the pathological process as suggested by Aschauer et al's (2014) study.
As mammals, humans lost the ability to synthesise their own ascorbic acid 200 million years ago (Marik et al, 2018) and rely on dietary intake to provide an adequate supply, which is relatively easily achieved during periods of health. However, with the support of previous studies, Aschauer et al (2014) claim that the amount of ascorbic acid required to prevent inactivation of key mediators of endothelium cell functions by reactive oxidant species during acute inflammation cannot be attained by oral supplementation, a claim also supported by Fowler et al (2014).
IV administration of ascorbic acid as detailed in this study was demonstrated to be a successful method for adequate delivery of ascorbate, with no notable side effects recorded by Aschauer et al (2014); this supports it being a safe means of introducing the antioxidant for therapy in a parenteral route that paramedics are trained to use (Seymour, 2010). However, this study included only people who were, in essence, exposed to only a self-limiting inflammatory-inducing dose of LPS while in an initial healthy state (Aschauer et al, 2014). Therefore, because people with confounding comorbidities, such as immunosuppression—often a feature of those with severe sepsis (Tupchong 2014), as seen by paramedics (Kirby 2013)—were not included, further research would be needed to support its advocacy.
Organ dysfunction is often a result of a compromised immune system (Vincent et al, 2011). Those who deteriorate further in the later stages of sepsis are consequently at risk of this (Prucha et al, 2017). These patients are often transferred to ICU with subnormal plasma ascorbic acid levels (Marik et al, 2017); Fowler et al (2014) agree, adding that this deficiency persistently amplifies tissue injury, accelerating subsequent organ dysfunction.
The SOFA score was developed to clinically measure this (Moreno, 1999) within ICU and was used by Fowler et al (2014) during a phase I clinical trial, which explored the effects of restoring ascorbic acid plasma level as a therapeutic adjunct in the treatment of severe sepsis. Each patient included in this study was initially scored using the SOFA scale to confirm the multiple organ failure. A higher dose of 200 mg/kg of ascorbic acid was administered to half of the blinded treatment group over 24 hours for 4 consecutive days, while a lower dose of 50 mg/kg was administered to the participants in the treatment group. Those in the randomised placebo group received no ascorbic acid, only the accompanying 5% dextrose infusion used in the treatment group (Fowler et al, 2014).
Once again, those receiving the ascorbic acid in addition to standard ICU interventions, including antibiotics, fluid resuscitation and vasopressor medication (Kirby, 2013), saw consistent decreases in their SOFA scores; the degree of reduction was comparative to the dose of ascorbic acid administered. This was in contrast to those in the placebo group regimen, who all experienced increased in their SOFA score. Fowler et al (2014) stated that any rise following initial treatment has historically predicted mortality rates of at least 50%, thereby demonstrating that IV ascorbic acid therapy reduces sepsis-induced organ injury, attenuating the deterioration of endothelial injury (Aschauer et al, 2014).
Fowler et al's (2014) study also reinforces ascorbic acid's effectiveness in cases of sustained inflammation in severely septic patients, which had previously been unchallenged in the aforementioned study (Aschauer et al, 2014).
However, there are obvious limitations and variabilities that could reduce the value of these conclusions.
First is the use of the SOFA score to determine probable mortality rates and therefore measure improvement. Although it was argued to be a robust measure by Fowler et al (2014) and others such as Marik et al (2017), it could have positively skewed the results. This is because a recent systematic review and meta-analysis of its use, conducted by Fernando et al (2018) involving a multitude of cohort studies of various sizes, concluded that the SOFA scoring system had poor sensitivity and only moderate specificity for short-term mortality rates, in line with the 4-day period assessed by Fowler et al (2014).
Second, the use of small participant groups in a study, especially when restricted to a single setting, has its limitations and provides potential selection bias (Long, 2013)—although this factor has been partially mitigated by using a double-blind randomised control trial, sustaining some associated quality (Brownell et al, 2013). However, supported by earlier findings, there was agreement upon the safety aspect and tolerability of the IV therapy, and this trial also said there were no notable side effects (Fowler et al, 2014).
At this stage, the potential benefits of administering ascorbic acid intravenously are promising in addressing vascular tissue injury in the early stages of sepsis and preventing organ damage in the more advanced stage of severe sepsis, both of which are encountered in the prehospital setting (Smyth et al, 2016). This makes a good argument for perhaps having a prophylactic bolus initiated by clinicians such as paramedics in this environment, without any recorded adverse reactions. This is also supported by the use of varying dosages in the previous two studies (Aschauer, 2014; Fowler, 2014).
With regards to the use of the SOFA scoring system, Smyth et al (2016) argue, after carrying out a systematic review, that the identification of the varying stages of sepsis in the prehospital environment is poor and screening tools have little clinical value in an emergency. That said, Fernando et al (2018) advocate the use of the systemic inflammatory response syndrome criteria, which have superior sensitivity, supporting its use for prompting treatment; this could therefore be adopted for any future prehospital-related studies.
Three decades of clinical trials, including 100 phase II and III trials, have yet to demonstrate a valid pharmacological agent that improves the outcome of those experiencing septic shock (Marik et al, 2017). A possible explanation and a running theme is a lack of ascorbic acid in circulating plasma.
In their retrospective before-after study, Marik et al (2017) demonstrated that ascorbic acid compared with previously trialled agents has markedly better effects in reversing vasculature impairment during shock and reducing mortality. There were 96 patients involved in the trial, with no significant baseline differences; all 47 who received the ascorbic acid infusion survived and were discharged from ICU compared with a mortality rate of 40.4% in the control group.
Unlike in the previous studies, the full spectrum of septic patients was observed. Therefore, their (PCT) levels were monitored (Marik et al, 2018). PCT can be used to quantify the level of infection (Lee, 2013), with a higher PCT level indicating amplified toxin levels and therefore a greater degree of infection. This is perhaps a better numeric to monitor outcomes than the SOFA score alone.
Although Fowler et al (2014) tested PCT levels, they were predominately used to set criteria for inclusion into their study, with a focus on SOFA scores as an indicator for their outcomes. However, as both PCT levels and SOFA scores were used, this potentially added value to the outcomes.
Hydrocortisone and thiamine were added to the infusion; both have been studied as singular entities in the treatment of septic shock and have been shown to effect little change in mortality rates (Barabutis et al, 2017). Marik et al (2017) said they were added only because recent studies had suggested they had a synergistic relationship with ascorbic acid (Barabutis et al, 2017).
Given that the differences between each treatment group and their placebo counterparts showed statistical significance (P<0.05) in all reviewed studies, it would be difficult to argue against the correlation between the direct, beneficial effect of IV-administered ascorbic acid for improving outcomes in those with sepsis (Aschauer et al, 2014; Fowler et al, 2014; Marik et al, 2017).
Conclusion
Current therapies used in the treatment of sepsis predominately aim to control inflammation and vascular function, while appearing to neglect subnormal levels of a key antioxidant; addressing this could complement current interventions.
Following this review of three recent randomised controlled trials, it can be suggested that IV administration of ascorbic acid has the potential to reduce sepsis-related mortality. This would be initially by reducing endothelial dysfunction, which could subsequently help prevent further vascular tissue injury and therefore substantial organ damage, averting potential sepsis-induced shock.
Furthermore, with no significant evidence of risks involving ascorbic acid therapy, there is a potential argument for administering it prophylactically in the prehospital environment by able clinicians such as paramedics to prevent deterioration by early intervention. However, as no current studies have been conducted in this setting, further research would be required before any trials could be run to ascertain any reasonable clinical value.

