This article gives a narrative review of a clinical case, puts it into the context of current research evidence and provides a discursive review of emergency prehospital clinical practice. This incident occurred before the coronavirus pandemic so draws on the clinical guidelines used at the time.
Case presentation
The patient, a woman in her late 20s, suddenly collapsed at home without warning and was found to be unconscious and not breathing by her partner. Immediate basic life support was given via ambulance telephony advice.
A paramedic team leader situated nearby was dispatched along with a dual-crewed ambulance. The team leader was the first to arrive on scene, seven minutes after the 999 call, and immediately established that the patient was in cardiac arrest, which was confirmed by no respiratory or cardiac output. Furthermore, an electrocardiogram (ECG) reading showed ventricular fibrillation (VF). One defibrillation shock was administered, and cardiopulmonary resuscitation (CPR) recommenced. The patient's airway was managed using an oropharyngeal (OP) airway and bag-valve mask, which was sufficient in enabling adequate ventilatory support to be provided (Deakin et al, 2015).
A medical history revealed a previous diagnosis of epilepsy but with no seizures for years, as well as a recent diagnosis of hyperthyroidism for which medication had been prescribed.
Causative factors for the cardiac arrest were considered, including reversible causes (4Hs: hypoxia; hypovolaemia; hyperkalaemia/hypokalemia/hypoglycaemia/hypocalcaemia (and other metabolic disturbances); and hypothermia; and the 4Ts: thrombosis (coronary or pulmonary); tension pneumothorax; tamponade (cardiac); and toxins).
The patient was not pregnant nor post-partum but had a young child aged approximately 2 years. She had no history suggestive of headaches/intracranial bleed, meningococcal illness, deep-vein thrombosis or pulmonary thrombosis. She had reported no chest pain and did not have any cardiac issues or abdominal complaints. Her abdomen was soft with no distension or pulsatile mass. There had been no recent stress, depression or grief within the family. There was nothing suggestive of medication or illegal drugs overdose. No recent minor illnesses were reported. In addition, a brief physical assessment revealed no obvious trauma, no peripheral oedema and no other clues to indicate a reason for cardiovascular collapse.
Prescribed medications were difficult to establish from the partner. It later became apparent that the patient had not been compliant with her prescription regimen of propanolol and other (unknown) medication for hyperthyroidism.
Prehospital clinical management
Return of spontaneous circulation (ROSC) was achieved after the first defibrillation. The patient continued to tolerate the OP airway while her level of consciousness increased (Glasgow Coma Scale (GCS) 5: eye response: 1; verbal response: 2; motor response: 2). She was pale and sweaty. Her pupils were dilated, both at 8 mm and not reactive to light.
Five minutes after ROSC, a 12-lead ECG identified a supraventricular tachycardia of 169 beats per minute with no ST-elevated myocardial infarction noted, respiratory rate of 24 per minute, oxygen saturations 100% on oxygen, end-tidal carbon dioxide 2.8 EtCO2, chest sounds bilaterally equal with mild crackles to both anterior bases, blood pressure 153/91 mmHg, temperature 35.6°C and a blood glucose reading of 9.6 mmol/l.
Neurologically, the patient became increasingly combative and cerebrally agitated as may be noted after ROSC (Deakin et al, 2015). As such, it became difficult to manage her safely within the confines of the small room she was in. Therefore, management included requesting helicopter emergency medical services (HEMS) attendance for advanced clinical support and guidance from the medical incident adviser regarding the level of sedation via intravenous (IV) diazepam (Griffin et al, 2013). While diazepam is noted within clinical guidelines, it is currently not indicated for paramedic administration in such cases (Association for Ambulance Chief Executives (AACE), 2019). However, Resuscitation Council UK guidelines (Deakin et al, 2015) recommend its use in combative patients.
The medical incident adviser permitted titrated diazepam to be given. Increments of 2.5 mg doses were administered. After the initial dose, the patient responded well in becoming less agitated, but the therapeutic effects wore off after 5 minutes. A second 2.5 mg dose was given, which had limited effect. The patient continued to become increasingly agitated and combative.
She was not responsive to verbal commands but did respond to painful stimulus with decorticate flexor posturing indicated by stiffening and ‘internal rotation of her upper limbs and extensor posturing of the lower limbs' (Munakomi and Das, 2020). This primitive reflex response is clinically significant as it indicates gross neurological pathology and is often observed in patients with significant traumatic brain injury, with severe metabolic imbalance or diffuse cerebral hypoxia (Macyszyn and Grady, 2012).
Given the circumstances and potential risk of serious injury to the patient, a third dose of 2.5 mg diazepam IV was administered with reasonable effect, lasting approximately 5–10 minutes.
The HEMS enhanced critical care team were dispatched by land as weather constraints restricted them from flying. Because of the remote geographical location of the incident from the HEMS base and the patient's critical presentation, a rendezvous point was arranged halfway between the scene and the receiving hospital, which was 20 miles away).
On arrival of the HEMS team, the ground paramedics gave a handover indicating that the patient remained agitated with marked trismus. Her respirations had become irregular with a rate of 15–40 breaths per minute but all other observations were as previously noted.
To provide safe onward care, the critical care paramedics gave the patient sedation following telephony consultation with the medical incident adviser. Midazolam, a pharmacological sedative that is noted to have limited haemodynamic influence (Barends et al, 2017), has several indications for administration within the prehospital field including post-resuscitation agitation (Rice et al, 2016).
With a rapid onset of action time of 2–5 minutes, 1 mg of midazolam IV was given to good effect and the patient remained sedated throughout transit. Although a prehospital emergency anaesthetic was indicated for airway compromise and impaired level of consciousness, the HEMS team in this instance was made up of two critical care paramedics, so this was beyond their scope of practice. No British Association for Immediate Care (BASICS) doctors were available within the vicinity.'.
The HEMS team travelled with the land ambulance crew to the emergency department with a working diagnosis of hypoxic brain injury secondary to an unknown cause of cardiac arrest. At hospital, the patient underwent rapid sequence induction. Following induction, the patient developed a narrow complex tachycardia of approximately 200–220 bpm and was given a DC cardioversion, which reverted the rhythm to a sinus tachycardia.
The patient's blood results were grossly normal with the exception of her free thyroxine levels which were elevated at 81.8 pmol/l. A computerised tomography scan of her head revealed no concerning conditions. She was ventilated and transferred to the intensive care unit (ICU), where she received treatment for thyrotoxicosis, which was believed to have caused her cardiac arrest secondary to non-compliance with hyperthyroidism medication. Twenty-four hours later, the patient was extubated and regained a full level of consciousness and had normal neurological function. She was later discharged with outpatient follow-up.
Discussion
Survival from out-of-hospital cardiac arrest is rare (Voss et al, 2014) and depends upon accurate diagnosis followed by rapid treatment.
The leading causes of cardiac arrest in women in the UK in their 20s were most recently recorded as suicide, accidental poisoning, transportation incidents, breast cancer and liver cirrhosis (Public Health England, 2017). Death from endocrine disorders was not listed as a significant cause of death within any age range. In the presenting case, this patient's VF cardiac arrest resulted from acute thyrotoxicosis; this is an extremely rare endocrinological syndrome associated with high levels of mortality. A literature review has identified that, internationally, very few published clinical case reports have detailed such incidents (Nakashima et al, 2014).
The pathophysiology of thyrotoxicosis is best understood in the contexts of normal thyroid physiology and the clinical condition of hyperthyroidism.
Anatomy and physiology
The thyroid is a small two-lobed gland weighing approximately 15–25 g that is situated in the anterior aspect of the neck, just above the trachea (De Groot and Jameson, 2013).
Hormone production within the gland is stimulated by thyroid-stimulating hormone (TSH) which is released from the anterior pituitary gland (Warrell et al 2016). In response to TSH, the thyroid produces thyroxine and triiodothyronine (T3) and releases them into the bloodstream. Thyroxine remains inactive until it is converted into triiodothyronine in the kidneys and liver. These hormones are critical to the development and metabolic functioning of cells and homeostasis, and they influence basal metabolic processes. Through a negative feedback mechanism, these hormones inhibit TSH production when levels of thyroxine and triiodothyronine become raised. They also influence the hypothalamic production of thyrotropin-releasing hormone, which stimulates the pituitary gland to produce TSH. Figure 1 shows the negative feedback regulation of thyroid hormones (Waugh and Grant, 2006).
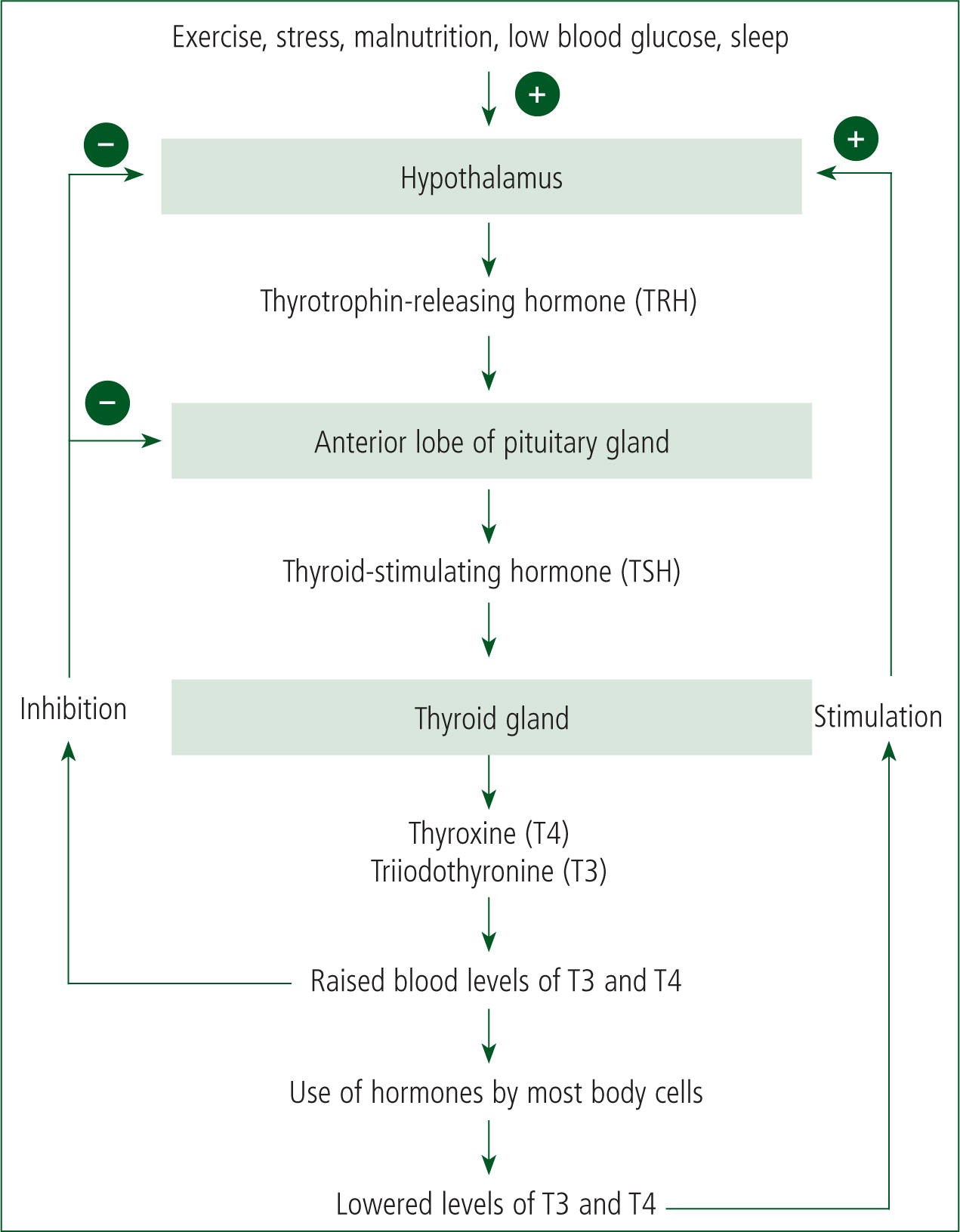
Hyperthyroidism
Disordered physiological processes associated with disease or injury to the thyroid gland or the hormones it produces (which may result from too little TSH may lead to a hormonal imbalance where excess hormones are produced and circulated in the bloodstream (NHS, 2019a). Critically, this pathophysiological process, called hyperthyroidism, adversely affects homeostasis, with the body's metabolism being accelerated.
While some patients are asymptomatic, this disease may give rise to a number of clinical signs and symptoms such as general fatigue, weakness, breathlessness, heat intolerance, weight loss and psycho-emotional changes such as mood instability (NHS, 2019a).
Hyperthyroidism is also associated with electrophysiological changes that affect the generation and conduction of cardiac impulses and contractility of the heart, resulting in an increased stroke volume and a risk of arrhythmias (Kahalay and Dillman, 2005; Nai et al, 2018).
Diseases that may cause hyperthyroidism include Graves' disease, where excess stimulation of TSH receptors causes increased hormone secretion, and lymphocytic thyroiditis, which is a destructive autoimmune condition that causes excess release of stored thyroid hormone. The main types and causes of hyperthyroidism are shown in Table 1.
| Clinical type | Biochemical pathology and aetiology |
|---|---|
| Primary hyperthyroidism | Abnormal functioning of the thyroid gland such as in Graves' disease or thyroiditis, which results in high levels of hormones being produced that interfere with the normal feedback control mechanism |
| Overt hyperthyroidism | Levels of circulating thyroid-stimulating hormone (TSH) are suppressed or undetectable. In addition, free thyroxine (FT4) or triiodothyronine (T3) are elevated. Patients are usually more symptomatic than those experiencing subclinical hyperthyroidism. The most common cause of this presentation is Graves' disease, adnenoma, toxic nodular goitre, thyrotoxicosis or excess thyroid hormone ingestion |
| Subclinical hyperthyroidism | Low or undetectable levels of TSH in the blood. Serum levels of T4 and T3 are within normal levels. This condition is caused by disease of the thyroid (such as a goitre, Graves' disease or thyroiditis) or exogenous excess thyroid hormone ingestion, such as excess use of or too high a prescribed dose of levothyroxine. Patients can be asymptomatic |
| Secondary hyperthyroidism | The thyroid gland is overstimulated by excess TSH being produced in the pituitary gland, excess TSH-secreting tumour factor or, sometimes, overproduction of thyrotropin-releasing hormone (TRH) by the hypothalamus or TRH-secretion factor. Cancer is the most likely cause |
| Thyrotoxicosis without hyperthyroidism | Excess thyroid hormone may be derived from drugs such as levothyroxine, or when a damaged thyroid gland releases stored thyroid hormone into circulation (Warrell et al, 2016) |
Chaker et al (2016) report that ‘both overt and subclinical hyperthyroidism are associated with hypertension, dyslipidaemia and coronary heart disease (CHD), whereas an excess of thyroid hormone, subclinical and overt hyperthyroidism increase the risk of atrial fibrillation, coronary heart disease and heart failure’.
The vast majority of diagnoses of hyperthyroidism are made in general practice and referred for endocrinological review. However, symptoms persist in some patients despite pharmaceutical treatment (which is usually with prescribed thionamides such as carbimazole or propylthiouracil), or patients do not seek medical advice. Often, the only patients who are admitted to hospital are those with decompensated cardiac failure, who tend to be older adults with rapid atrial fibrillation (AF) (Reddy et al, 2017).
Thyrotoxicosis and thyroid storm
If hyperthyroidism is not managed or treated, significant overproduction and excessive release of circulating thyroid hormones may result in acute thyrotoxicosis, a rare clinical syndrome resulting in a dangerously high metabolic rate. Acute thyrotoxicosis may also be referred to as a ‘thyroid storm’ or ‘thyrotoxic crisis’ (Warrell et al, 2016). This condition is a medical emergency and requires hospital admission for treatment.
The literature indicates that the pathophysiological processes leading to thyrotoxicosis are still not fully understood. However, there are a number of accepted clinical systemic manifestations of the syndrome (Table 2).
| System | Clinical presentation |
|---|---|
| Central nervous | Tremors, fatigue, delirium, severe confusion, agitation, inability to concentrate, myopathy |
| Cardiovascular | Tachycardia, arrhythmias, systolic hypertension with wide pulse pressure leading to congestive heart failure, chest pain, palpitations |
| Respiratory | Increased respiratory rate, dyspnoea, respiratory compromise, pleural effusion |
| Gastrointestinal | Loose stools, increased bowel movements, diarrhoea, abdominal pain, jaundice, vomiting |
| Genitourinary | Polyuria, lighter menstruation and longer cycles in women and girls, amenorrhoea. |
| Psychological | Anxiety, psychosis, psychotic behaviours, agitation, changes in mood, paranoia, panic attacks |
| Other | Bulging eyes, high temperature, heat intolerance, profuse sweating (diaphoresis) leading to dehydration and electrolyte imbalance, weight loss despite good/increased appetite, thyroid gland enlargement, dermopathy causing thickening of the skin of the lower legs, urticaria, difficulty sleeping, peripheral oedema |
As noted, it is clinically recognised that the heart is particularly sensitive to the hypermetabolic state induced by a dysregulated thyroid hormone imbalance and this has a considerable impact upon cardiac homeostasis. A dangerous rise in circulating thyroid hormones has a direct beta-adrenergic effect on the myocardium, leading to increased sympathetic nervous system activity. In thyrotoxicosis, this is likely to result in a range of arrhythmias (Owecki et al 2006; van Noord et al, 2008; Ueno et al, 2010; Chaker et al, 2016), including supraventricular tachycardia, Brugada syndrome, atrial fibrillation, atrial flutter (Carroll and Matfin, 2010) or VF (Nai et al, 2018: 351). Tachycardia, cardiovascular compromise and fever are the most common systemic findings associated with thyrotoxicosis (Burmeister, 2019). Furthermore, decreased contractility of the myocardium and increases in ejection fraction and cardiac output are predictive of congestive cardiac failure with associated peripheral oedema and respiratory compromise. These clinical manifestations are associated with poor outcomes.
The overall epidemiological incidence of thyrotoxicosis in the UK is ‘around 2% in women’, ‘0.2% in men’ and ‘affects 1 to 2 children per 10 000’ (National Institute for Health and Care Excellence (NICE), 2019). Graves' disease is the primary aetiology ‘accounting for 60–80% of cases’ (NICE, 2019). Other comorbidities and issues may be factors in the development of this life-threatening condition. These include:
Thyrotoxicosis is often triggered by the cumulative effect of such factors leading to an inability to maintain metabolic and cardiovascular homeostasis.
If untreated or if there is poor compliance with hyperthyroid medication (Matfin, 2018), thyrotoxicosis is likely to result in multiorgan dysfunction and has a high rate of associated mortality from early cardiac repolarisation and prolonged QT interval leading to VF. However, precise data on the numbers of thyrotoxicosis-related cardiac arrests with ROSC in either the prehospital or hospital settings appear to be absent from the literature. Furthermore, the exact mortality rate is unknown but thought to be high (Carroll and Matfin, 2010).
This condition is therefore an endocrine emergency requiring immediate intervention in hospital. It is undoubtedly early recognition and prompt treatment that result in a positive outcome for these patients.
Case discussion
It was not known in this case if the patient had displayed any predisposing symptoms before her collapse. However, it was noted that she had stopped taking her prescribed hyperthyroidism medication.
Thyrotoxicosis was diagnosed as the likely cause of the VF cardiac arrest. This was confirmed upon receipt of her blood results, which indicated that her blood levels of TSH were low <0.01 mU/litre and T4 levels were very high at 81.8 pmol/litre, in comparison to the normal ranges shown in Table 3. Importantly, raised T4 levels in the blood are associated with an increased risk of sudden cardiac death resulting from cardiac arrhythmias (Chaker et al, 2016).
| Thyroid-stimulating hormone (TSH) | 0.4–4.0 mU/litre |
| Free thyroxine (FT4) | 9.0–25.0 pmol/litre |
| Free triiodothyronine (FT3) | 3.5–7.8 pmol/litre |
While the literature is limited, three studies are of relevance and interest. Most recently, a retrospective study by Tamatea et al (2019) highlighted the rarity of thyrotoxic-related cardiac conditions. This research sought to understand the prevalence and type of thyrotoxicosis-associated heart disease in a population of Maori patients in New Zealand. Overall, the study, which spanned 7 years (2005–2012), recorded a total of 35 337 cardiac-related hospital admissions; only 72 of these patients were identified in having developed cardiac complications caused by thyrotoxicosis, which indicates the rarity of this condition.
Of these 72 patients, 32 were admitted for dysrhythmias, 12 for ischaemia, 11 for cardiac failure; 17 had mixed cardiac disease; 47 patients presented with a tachyarrhythmia (four with AF, one with ventricular tachycardia, 27 with heart failure and 16 with cardiac ischaemia).
This study, however, did not explore cases of cardiac arrest induced by thyrotoxicosis. Indeed, only two recent, credible cases were identified within our literature review.
Nakashima et al (2014) reported the case of a 23-year-old man who collapsed while running. Initially, he presented in a pulseless electrical activity (PEA) rhythm. After the administration of epinephrine and atropine, the patient developed VF. Defibrillation was provided, and sinus rhythm returned. There was no evidence of any cardiac event in ECGs, blood tests or heart scans. The TSH level was <0.005, with free T4 at 5.03 and free T3 at >30.00. After treatment for the thyroid storm and hyperthyroidism, the patient stabilised and exhibited no recurrence of VF or other cardiac arrest rhythms. It was reported that a thyroid storm may cause early repolarisation and coronary artery spasm. However, in this case, the patient smoked, which could have been a contributory factor (Ando et al, 2011).
A further case reported by Ueno (2010) highlighted a patient who was being monitored in an ICU.
This patient had been reviewed for nausea, vomiting and diarrhoea. Following an examination and blood testing, it was found that he had reduced TSH levels and increased free T3 and increased free T4 levels, with a positive TSH receptor antibody. He was medicated to reduce these levels but this was subsequently stopped because a drug-induced liver function disorder developed.
The patient's condition worsened, with the appearance of delirium, paroxysmal atrial fibrillation and respiratory failure. The patient was sedated and admitted to ICU. On day 1 of admission, he developed AF and, on day 4, he developed VF. After defibrillation, sinus rhythm was restored. This case reports finding ECG changes with J-point elevations in the inferior leads and V3–V6, which had become more pronounced throughout the patient's admission. On day 5, the patient died from multiple organ failure.
While a small number of other case studies are reported, there appears to be very little understanding of the detailed pathophysiology resulting in VF arrest in response to a thyroid storm (Wei et al, 1979; Jao et al, 2004; Chatterjee et al, 2009; Ueno et al, 2010; Ando et al, 2011; Brooks et al, 2013; Nakashima et al, 2014; Kobayashi et al, 2015).
In this clinical case, all attending crew were satisfied that treatment was provided to the best of their abilities within their scope of practice. Thyrotoxicosis was not a cause of cardiac arrest that could excluded out in the field, particularly given the patient's diagnosis of hyperthyroidism. Blood testing for endocrine imbalance is not widely carried out in the UK prehospital arena so any clinical indicators must be drawn from the history and presentation alone.
However, even if this is considered as a cause, primary management should focus on immediate resuscitation and transportation to a suitable receiving facility (Resuscitation Council UK, 2015). Indeed, what this case study and that by Nakashima et al (2014) indicate is that, while this condition is an extraordinary cause of cardiac arrest, it is potentially reversible with a good outcome.
Conclusion
Cardiac arrest secondary to thyrotoxicosis is a rare event. Such patients experiencing out-of-hospital cardiac arrest should be managed in line with current best practice guidance.
In this instance, identifying or treating the cause in the prehospital environment was not possible. However, this case demonstrates that an early call for help, bystander CPR, early defibrillation and post-resuscitation care are of vital importance in the ‘chain of survival’ as described in the UK Resuscitation Council Guidelines (2015). Gaining an accurate history is of secondary importance.
From examining the literature, the authors have concluded that there is little documented understanding or evidence of this syndrome in the pathophysiological context of VF.
This case report demonstrates that this patient was successfully resuscitated following a VF arrest secondary to thyrotoxicosis and survived to discharge with normal neurological function. No follow up was made after discharge.

