Gamma-hydroxybutyrate (GHB) was first used in humans by the French pharmaceutical industry in the 1960s (Laborit et al, 1960). Originally used as a gamma aminobutyric acid (GABA)-ergic central nervous system (CNS) depressant, it induces neurotoxicity in animals (Ainslie et al, 2016).
From the early 1990s, it became an attractive drug of recreational abuse because of its low cost and widespread availability (Nicholson and Balster, 2001). GHB and its precursor gamma-butyrolactone (GBL) are now witnessing widespread illegal use in clubs for gay men and the chemsex scene (European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), 2008).
Few studies focus on the prehospital management of GHB acute toxicity. The purpose of this literature review is to broaden the prehospital clinician's knowledge and serve as a guide for their future encounters.
Background
Quality of the research evidence
During background reading, mistakes were found in some publications. Busardò and Jones (2015) incorrectly illustrated an image portraying the pharmacodynamics of GHB. This error in a peer-reviewed publication reflects the complexity of the topic. Unfortunately, the evidence base for the management of GHB acute toxicity predominantly includes retrospective observational studies. Despite this, the evidence from available sources is worth exploring.
Street names
GHB has various street names including liquid ecstasy, G and Gina (Mokhlesi et al, 2004).
Legal status
The newly amended Misuse of Drugs Act 1971 now classifies GHB and GBL as class B drugs; they are schedule 2 substances under the Misuse of Drugs Regulations 2001.
Epidemiology
The EMCDDA estimated the use of ketamine, GHB and poppers (alkyl nitrites including amyl nitrite and isobutyl nitrite) among young adults (15–34 years) to be 0.6% of the population in 2015 (EMCDDA, 2017).
However, despite its low prevalence compared to other recreational drugs, the European Drug Emergencies Network stated that GHB was the fourth most prevalent substance to induce acute drug toxicity over 2013–2014 (Dines et al, 2015).
User demographics
GHB use is mainly reported in nightclubs, sex parties and saunas (Halkitis and Palamarr, 2006). Of the 61 GHB-associated deaths identified by Hockenhull et al (2017) between 2011 and 2015 in London, 98% were in men with a median age of 37. Drugs associated with chemsex were detected in 65% of these cases. It can be concluded that GHB use is concentrated among men who have sex with men.
Cost and purity
GHB is bought from street dealers or over the internet in volumes ranging from 125 ml to 10 litres. It costs approximately £20 per 250 ml bottle of 99% pure GBL (i.e. 8p per recreational dose) (Corkery, 2007). Normally, 1 ml of liquid contains 1 g of GHB (Miotto et al, 2001). A single dose of GHB is usually between 0.5 g and 5 g (Miotto et al, 2001).
Route of administration
The routes used to administer GHB include oral (the most common), insufflation, mucosal and injection (the least common) (Abdulrahim and Bowden-Jones, 2015).
The colourless liquid is commonly added to a beverage and the dose is often measured imprecisely using capfuls and eye droppers (Miotto et al, 2001). When this is undertaken in a dark and noisy nightclub, it is unsurprising that acute toxicity occurs.
User effects
The limited subacute adverse effects helped popularise GHB as a recreational drug (Mokhlesi et al, 2004). The dose-dependent effects include relaxation, euphoria, disinhibition, sociability, increased sexual arousal, dissociation and sedation (Busardò and Jones, 2015).
Acute toxicity
All GHB users risk acute toxicity (i.e. G-ing out) when large doses are consumed over short periods (Miró et al, 2017). The hazard profile is less favourable than that of most other psychoactive drugs (Griffiths and Johnson, 2005).
The CNS and respiratory depression seen in GHB use is short in duration. If other sedative-hypnotic drugs have not been consumed, rapid awakening from loss of consciousness with an uneventful recovery is typical; however, fluctuating periods of agitation have been reported (Abdulrahim and Bowden-Jones, 2015). Because of the steep, non-linear dose-response curve of GHB (Liechti et al, 2016), signs and symptoms vary depending on individual response, dose ingested and other substances consumed (Table 1).
| Dose | Clinical effects |
|---|---|
| <20 mg/kg | Anxiolytic effect, euphoria, short-term anterograde amnesia and hypotonia |
| 20–50 mg/kg | Drowsiness, dizziness, enuresis, hallucinations, sleep and myoclonus |
| >50 mg/kg | Coma, bradycardia, respiratory depression and death |
GHB is a dangerous recreational drug because of its extremely narrow therapeutic index. Respiratory failure resulting from respiratory depression is the cause of death in most cases (Zvosec et al, 2011).
Clinicians must be aware that the characteristic signs and symptoms of GHB acute toxicity may be masked in polydrug use. A comprehensive history, examination and investigation of environmental clues is therefore required.
Polydrug use
Alcohol, methylamphetamine, mephedrone, sildenafil and poppers are often taken with GHB (Hockenhull et al, 2017). This is not surprising given the user demographics.
Several studies have stated that GHB use alongside alcohol is more likely to result in severe toxicity (Galicia et al, 2019).
Withdrawal
Following dose reduction after prolonged use, withdrawal symptoms can be severe and are often a medical emergency (LeTourneau et al, 2008). Medical scoring systems, such as the Clinical Institute Withdrawal Assessment Alchohol Scale revised (CIWA-Ar) for alcohol withdrawal, may be of use. While this review will not focus on the harm associated with chronic use, it is important for prehospital and emergency medicine clinicians to be able to recognise and treat GHB withdrawal.
Harm reduction
The author would like to emphasise the importance of prehospital clinicians providing education to their patients. This can include advising them to: never mix GHB with alcohol; use a timer on their phone when they dose and never take more than 1 ml per hour; and consider buying 1 ml syringes from a pharmacy to get the dosing right.
Mortality
Both GHB acute toxicity and withdrawal have been associated with deaths despite relatively low levels of use.
GHB-associated deaths in London increased by 119% in 2015 compared to the previous year (Hockenhull et al, 2017). In 2018, GHB was mentioned on the death certificate in 28 deaths in England and Wales; however, the number is likely to be higher (Office for National Statistics, 2019).
Methods
Research question
The following research question was used: describe the optimum management of acute toxicity caused by recreational drug gamma-hydroxybutyrate.
Search strategy
A literature search was undertaken to identify the evidence surrounding the prehospital management of GHB acute toxicity. It was carried out on 5 March 2020 and used the Embase, Medline and PubMed databases.
Both Medical Subject Headings (MeSH) and free text searches were used. Boolean operators were applied to truncated synonyms of 4 hydroxybutyric acid, recreational drug, toxicity and emergency treatment. Limits were used so solely publications after 1990 were included. Additional resources and online books were searched for.
Screening and eligibility
Following the removal of duplicate records, the inclusion and exclusion criteria were applied. All inclusion criteria had to be met and a study that met any of the exclusion criteria was not included (Table 2).
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
Data collection and reporting
The relevant information from the publications was collected and reported qualitatively. Of the observational studies published after 2000 which provided an intubation rate for patients with suspected GHB acute toxicity, the criteria used and their limitations are listed where available.
Critical appraisal
Publications were independently assessed for their risk of bias. The author has highlighted the limitations of the evidence when relevant.
Findings
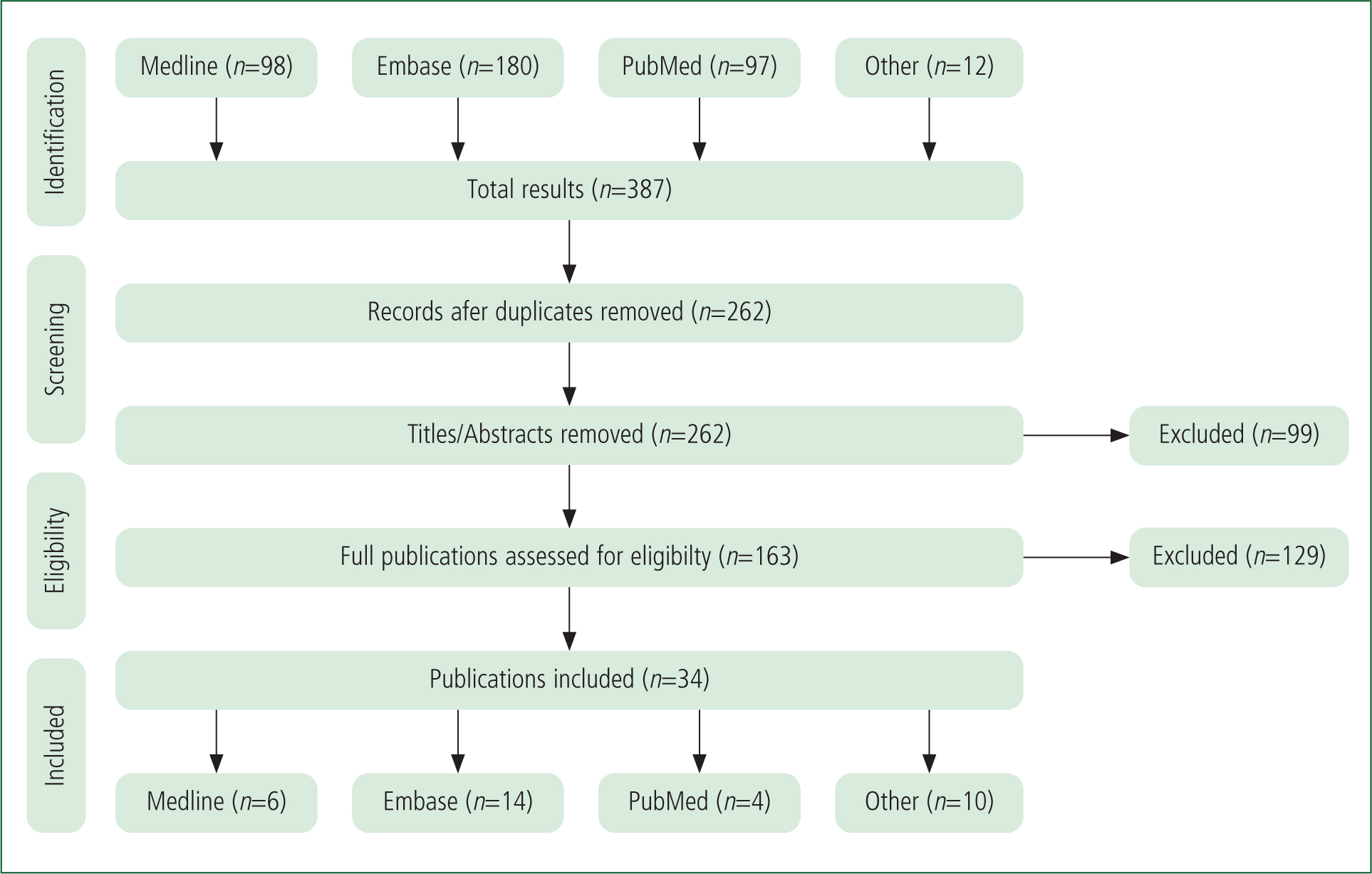
The literature search identified 387 results and, once duplicates had been removed, 262 results remained. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method (Moher et al, 2009) was used. Following screening and eligibility, 34 publications were considered suitable (Figure 1). Of these 34 publications, 12 provided an intubation rate for patients with suspected GHB acute toxicity after the year 2000.
Discussion
Pharmacology
A sedative-hypnotic drug, GHB is an endogenous precursor and metabolite of inhibitory neurotransmitter GABA (Bodson et al, 2008). Low doses produce euphoric effects; however, CNS and respiratory depression occur at higher doses as it is able to cross the blood–brain barrier (Mason and Kerns, 2002). GHB undergoes rapid absorption, distribution, metabolism and elimination (Wood et al, 2011).
High doses stimulate the G-protein-coupled GABA-B receptors and activate the mesolimbic dopaminergic pathway (Corkery, 2007). Once it has bound to GABA-B, it is predominantly metabolised in the liver and excreted as carbon dioxide and water through the lungs (Schep et al, 2012).
The maximum plasma concentration (Cmax) of GHB occurs 20–40 minutes after ingestion (Tmax) and it has a half-life of 30–50 minutes (Busardò and Jones, 2015). Because of this, the effects often occur 15–20 minutes after ingestion, peak at 30–60 minutes and last for 3–4 hours (Schep et al, 2012).
GBL and 1,4-butanediol are both precursors of GHB (Figure 2).
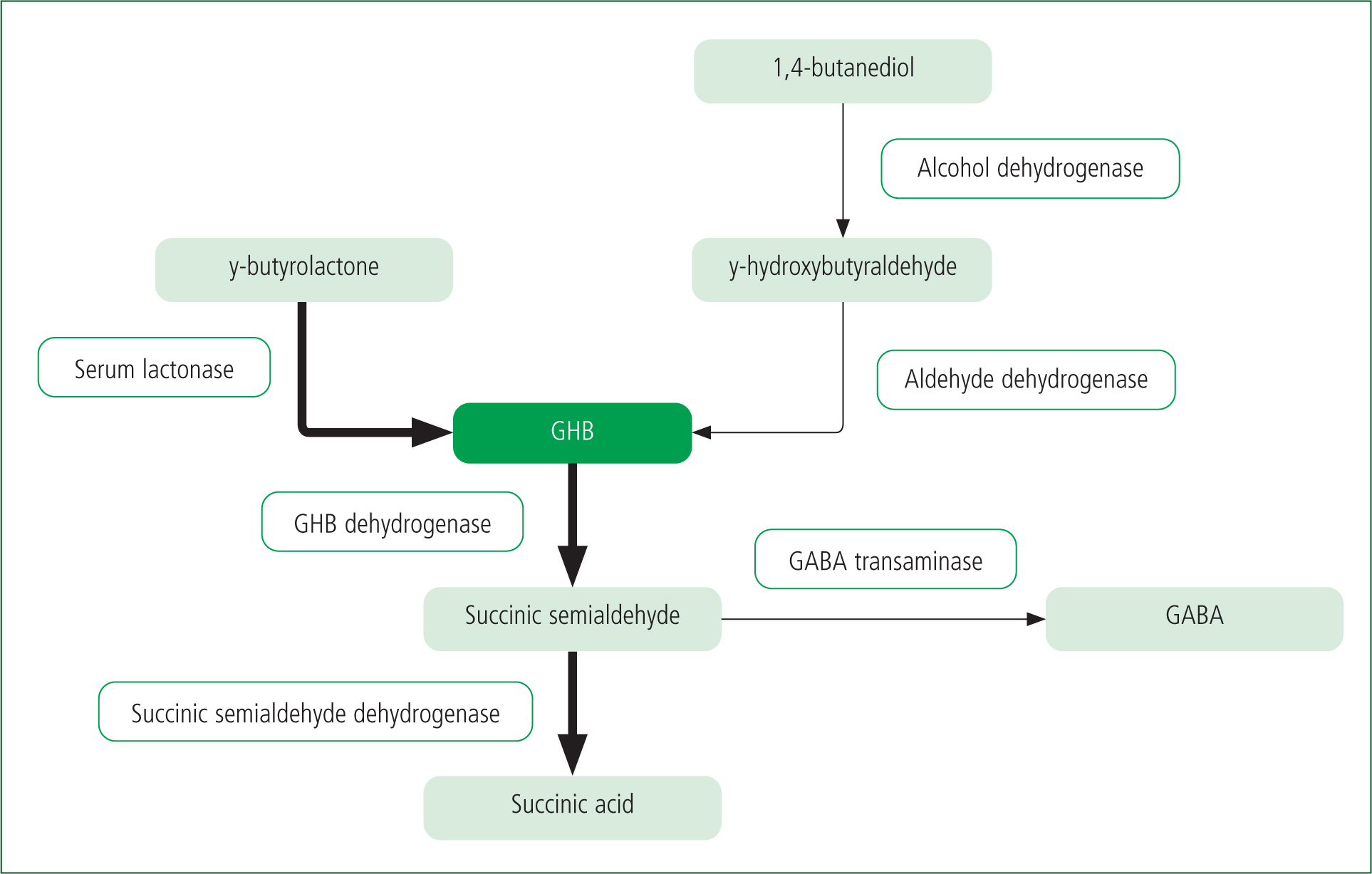
GBL is converted to GHB by serum lactonases (Teter and Guthrie, 2001). It is absorbed faster, has a more rapid onset of action and has a longer duration of effects, primarily because of its lipophilic nature (EMCDDA, 2002). 1,4-butanediol is partially oxidised by alcohol dehydrogenase to gamma-hydroxybutyraldehyde and then by aldehyde dehydrogenase to GHB (Snead and Gibson, 2005).
GHB is metabolised by GHB dehydrogenase into succinic semialdehyde (Bodson et al, 2008). It is then converted by succinic semialdehyde dehydrogenase into succinic acid, which enters the Krebs cycle and produces carbon dioxide and water (Mason and Kerns, 2002). The remainder of the GHB is converted into GABA by GHB dehydrogenase and GABA transaminase (Bodson et al, 2008).
Prehospital management of acute toxicity
Identification
Acute toxicity should be considered when a previously healthy young adult has a history of an acute change in mental status (Ricaurte and McCann, 2005). Differential diagnoses of GHB acute toxicity include medical, toxicological and traumatic causes of reduced consciousness.
Assessment
Sedative-hypnotic drugs typically result in a reduced level of consciousness, bradycardia and respiratory depression (Ricaurte and McCann, 2005).
Patients consuming GHB with other intoxicants present more with vomiting (15% versus 3%; P<0.001) and cardiovascular symptoms (5.3% versus 1.5%; P<0.05), have a greater need for treatment (59.8% versus 48.3%; P<0.01) and stay longer in the emergency department (11.3% versus 3.6%; P<0.01) (Miró et al, 2017).
These findings may not be generalisable as patient selection was not randomised; however, other studies have corroborated these results (Liechti et al, 2006; Liechti and Kupferschmidt, 2004). Polydrug use may therefore warrant more frequent observations.
Testing
GHB is not detected by most immunoassay drug screens (Palmer, 2004). Gas chromatography (GC) with mass spectrometry (MS) is used to determine GHB in blood and other fluids (Busardò and Jones, 2019). As of May 2012, the GC-MS urine illicit drugs screen now analyses for GHB (Hockenhull et al, 2017). Based on their extensive literature review, Busardò and Jones (2019) recommended an antemortem blood cut-off concentration of 5 mg/l and a urine cut-off concentration of 15 mg/l.
In most emergency departments, toxicological analysis for GHB is not available within a time frame that would influence management (Wood et al, 2011). The early diagnosis of acute toxicity is consequently based on the acknowledgment of the clinical toxidrome. A collateral history is often beneficial.
Antidotes
Currently, there are no effective antidotes for GHB overdose. Benzodiazepine antagonist flumazenil and opioid antagonist naloxone are ineffective (Andresen et al, 2008). Some studies have referred to the effective use of physostigmine (a parasympathomimetic) (Caldicott and Kuhn, 2001); however, its usefulness has been challenged (Zvosec et al, 2007).
Monocarboxylate transporter 1 (MCT1) is involved in the absorption, distribution, metabolism and elimination of GHB (Follman and Morris, 2019). Most recently, MCT1 inhibitors AZD3965 and AR-C155858 (Follman and Morris, 2019) have been shown to prevent the respiratory depression induced by GHB by increasing renal clearance in rats; however, research is needed in humans.
Clinical management
Management of GHB acute toxicity is supportive (Palmer, 2004). The airway must be initially managed non-invasively and supplemental oxygen titrated if required. It is essential to examine the oropharynx for burns as GHB is caustic (Teter and Guthrie, 2001). Continuous cardiorespiratory, blood pressure and pulse oximetry monitoring should be established and intravenous access obtained. Capillary glucose should be measured and corrected if required. A temperature should be obtained and non-invasive warming commenced if the patient is hypothermic.
Atropine and/or transcutaneous pacing should be used to treat symptomatic or severe bradycardia (Mason and Kerns, 2002). Antipsychotics are indicated in agitated patients. Benzodiazepines may be indicated in patients having seizures; however, these can worsen respiratory depression (Drasbek et al, 2006). Antiemetics should be considered and crystalloids, alongside vasopressors and/or inotropes, should be administered if the patient is hypotensive (Abdulrahim and Bowden-Jones, 2015).
If blood gas analysis reveals metabolic acidosis, intravenous fluid boluses, administration of bicarbonate, maintenance of normothermia and/or, if severe, haemodialysis may be indicated (Heytens et al, 2015). In cases of prolonged unconsciousness, rhabdomyolysis and/or kidney injury may mean haemodialysis is required (Palmer, 2004).
Other antidotes should be trialled to manage the effects of other agents in cases of clinical uncertainty. Chest X-ray is not usually required (Palmer, 2004).
Elimination
Because GHB is absorbed rapidly, activated charcoal is mostly not beneficial. However, rapid treatment should be considered in the context of unintentional intake by children, very high doses and polydrug use (Neijzen et al, 2012). Gastric lavage is also of limited value (Mason and Kerns, 2002).
Emesis should not be induced because of aspiration risk (Teter and Guthrie, 2001).
Airway management
Mechanical ventilation should be considered where there is hypoxia despite maximal supplemental oxygen or hypercapnia leading to metabolic acidosis (Abdulrahim and Bowden-Jones, 2015). Where these indicators are not present, consideration should be given to conservative airway management with simple manoeuvres alongside non-invasive end-tidal carbon dioxide monitoring as most patients make a rapid recovery (Summers and Glynne, 2007).
Intubation has been found to increase emergency department length of stay by 41% (95% CI (19%–65%)) and odds of hospital admission by 9.95 (95% CI (2.36–41.88)) in uncomplicated patients (Dietze et al, 2014). This study did attempt to minimise confounding variables; however, these findings could be attributed to the severity of the patient's initial presentation or the use of sedatives. There is evidence that patients with GHB acute toxicity can do well without intubation as long as aspiration can be prevented (Palmer, 2004). Lateral patient positioning and the use of suction alongside an antiemetic would be of use in these cases.
Only the most severe presentations of GHB acute toxicity generally require intubation, such as intentional overdoses of large quantities or polydrug use resulting in excessive haemodynamic instability requiring more complex interventions. Table 3 shows intubation rates for patients with suspected GHB acute toxicity for studies published after 2000. The criteria used by the studies and their limitations are listed where available.
| Study | Number of patients | Intubated (%) | Intubation criteria | Limitations |
|---|---|---|---|---|
| Miró et al (2002) | 104 | 3 |
|
|
| Couper et al (2004) | 146 | 23 |
|
|
| Liechti and Kupferschmidt (2004) | 141 | 13 |
|
|
| Anderson et al (2006) | 1331 | 16 |
|
Relied upon patient records because there was no ICD-10 coding |
| Liechti et al (2006) | 104 | 6 |
|
|
| Munir et al (2008) | 170 | 8 |
|
|
| Wood et al (2008) | 158 | 7 |
|
|
| Galicia et al (2011) | 505 | 2 |
|
|
| Dutch and Austin (2012) | 61 | 3 |
|
The aim to demonstrate on-site medical teams could successfully manage GHB acute toxicity may have influenced choice to refer to hospital |
| Dietze et al (2014) | 335 | 16 |
|
Did not define complicated |
| Miró et al (2017) | 710 | 6.9 |
|
|
| Galicia et al (2019) | 609 | 5.3 |
|
|
Intubation criteria and study limitations provided if available. Polydrug use differed between studies.
GCS: Glasgow Coma Scale; GHB: gamma-hydroxybutyrate; ICD-10 = International Classification of Diseases, 10th revision
Overall, the percentage of patients needing intubation is low. Couper et al (2004) perhaps reflect overcautious management as the indication criteria were not defined. The author had hoped that increased knowledge would promote confidence in more conservative management since Couper et al's (2004) study; however, the intubation rate has not decreased over time.
The evidence surrounding the indications for intubation is limited. The two most recent studies with two of the largest sample sizes failed to provide details of their criteria.
Galicia et al (2019) stated that all their patients requiring intubation had co-ingested alcohol (P<0.05). There appears to be some clinical consensus that patients should not be intubated on the basis of Glasgow Coma Scale score alone (as it has not been validated for use in toxicology) and that other clinical indications (such as vomiting or seizures) are required.
The decreasing availability of prehospital intubation in the UK because of a lack of evidence for its use in out-of-hospital cardiac arrest means it may not be an option for most clinicians. Other forms of mechanical ventilation could be of more relevance; for example, a laryngeal mask airway with or without nasogastric tube insertion in hospital should suffice for many patients.
If intubation is indicated, a rapid sequence induction technique should be used, followed by maintenance sedation (Palmer, 2004); however, the clinician should be mindful of cardiorespiratory and neurological depression.
Treatment outcome
GHB acute toxicity often results in rapid recovery. The median duration of symptoms has been found to be 2 hours requiring only a short period of observation (Liechti and Kupferschmidt, 2004). Non-intubated coma patients should remain in a coma for no longer than 4 hours. Additional causes should be considered if they remain in a coma for >4 hours and/or experience convulsions (Abdulrahim and Bowden-Jones, 2015).
Recommendations
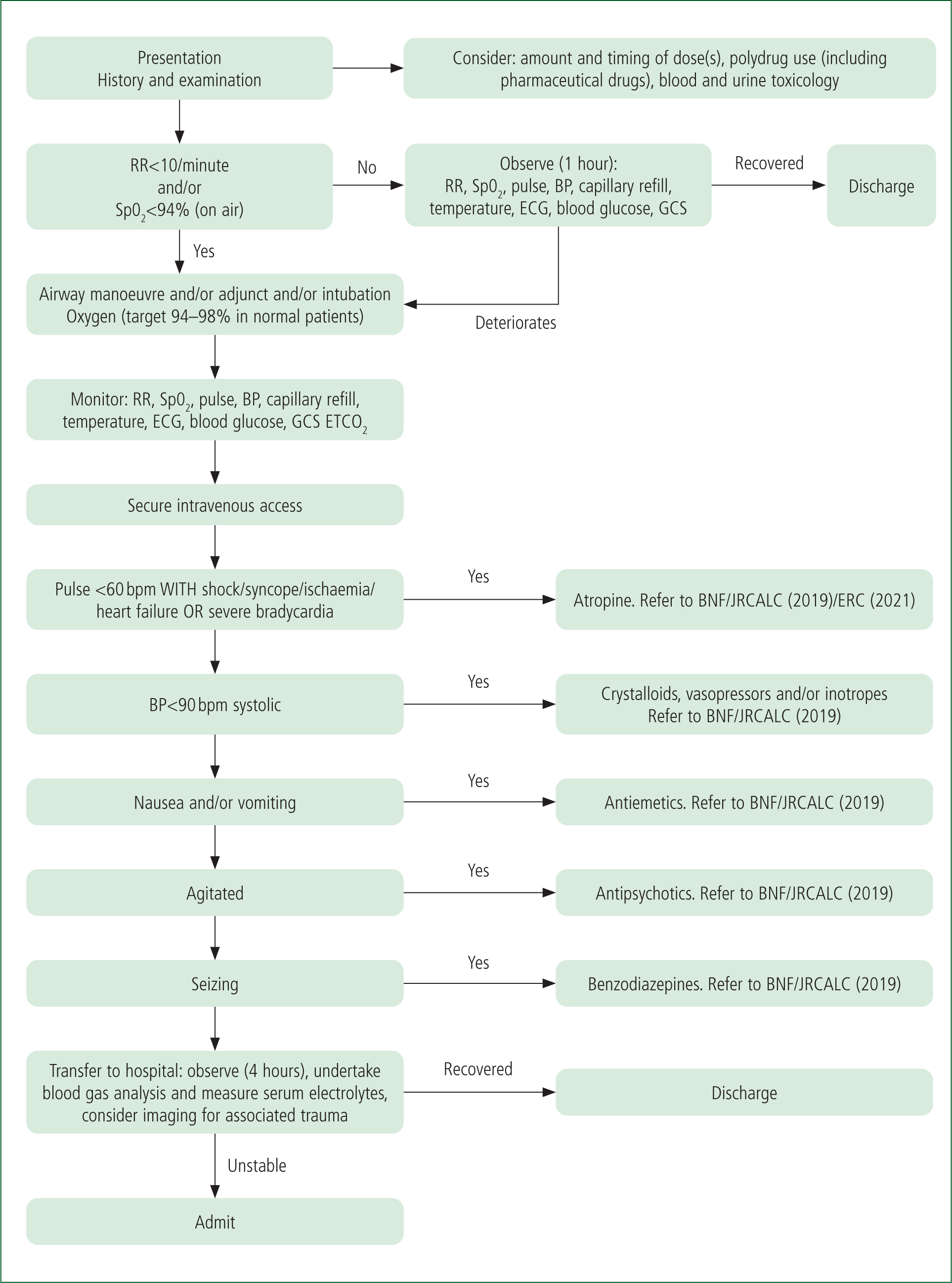
Considerable inconsistencies exist in the evidence base regarding how best to manage GHB acute toxicity in the prehospital setting.
A treatment algorithm (Figure 3), developed by the author, is based on the evidence available. Future work should focus on substantiating these recommendations.
Medical scoring system
Developed by the author, the medical scoring system shown in Table 4 is derived from the intubation criteria in Table 3.
| Parameter | Score | |
|---|---|---|
| Breathing | Periods of apnoea and/or significant refractory hypoxaemia or hypercarbia and/or severe acidosis | 3 |
| Bradypnoea | 1 | |
| Normal respiratory rate, oxygen saturation and blood gas analysis | 0 | |
| Circulation | Severe bradycardia | 3 |
| Mild bradycardia | 1 | |
| Normal pulse rate | 0 | |
| Disability | Emesis with GCS 3 | 3 |
| GCS 3 | 1 | |
| Rousable | 0 | |
| Other | Hypothermia | 1 |
| Polydrug use | 1 | |
| Uncertainty about patient history | 1 |
GHB: Gamma-hydroxybutyrate; GCS: Glasgow Coma Scale
In this derivation, a combined score of ≥3 indicates that mechanical ventilation may be required. This medical scoring system requires prospective validation before it is adopted into clinical practice.
Limitations
A finding or suspicion of GHB consumption is often based on the patient's history, and toxicological confirmation of its use is limited. The clinical picture may therefore be falsely attributed to GHB or another recreational drug.
Nevertheless, a study has found that toxicological analysis confirms the patient's history in 85% of cases (Galicia et al, 2011), and a negative result does not exclude its use because of its short half-life (Busardò and Jones, 2015).
Most publications also did not include patients seen outside the emergency department. GHB acute toxicity is self-limiting and may resolve in the prehospital setting.
The percentage of all patients requiring intubation would be lower than many of those stipulated in Table 3.
Conclusion
An inexpensive and readily available drug with a problematic toxidrome, GHB has a short but controversial history surrounding its increasing use, which will continue for the foreseeable future.
The treatment of GHB acute toxicity is generally supportive. Many emergency clinicians would tolerate profound unconsciousness in their patients if they were ventilating sufficiently as they will often make a rapid recovery. However, some clinical factors increase the likelihood of mechanical ventilation being needed. Hypoxaemia or hypercarbia on blood gas analysis that is not responding to conservative management should prompt a consideration for mechanical ventilation (with or without intubation).
Polydrug use increases both morbidity and mortality and should be treated more aggressively. This article highlights the continuing confusion surrounding the need for intubation. Further research is required to ascertain the indications, benefits and risks of intubating patients with GHB acute toxicity.

