This article will compare the use of two common anti-convulsants: midazolam (intravenous, intranasal and buccal formulations) and miazepam (intravenous and per-rectum formulations), in the treatment of prolonged tonic-clonic epileptic seizures. With epilepsy affecting approximately 50 million people worldwide and greater than half a million people within the United Kingdom alone (The Epilepsy Society, 2011), it is important to ensure that paramedics are providing the most effective treatment possible. This article will explore the pathophysiology of epilepsy and an acute seizure, specifically the tonic-clonic presentation as they require rapid therapeutic interventions by paramedics because prolonged seizures may result in complications for the patient. It will then explore the pharmacology of benzodiazepines and compare midazolam administered via intranasal, buccal and intravenous routes with intravenous and rectal diazepam, and the benefits of alternative routes of administration. With recent changes to legislation coming into effect it is important to consider the legal aspects of providing therapeutic interventions with benzodiazepines, which will be discussed later in this article.
Pathophysiology
Epilepsy is a homeostatic imbalance characterised by recurrent malfunctions in motor, sensory or psychological functions. These malfunctions are commonly described as a seizure (Tortora and Derrickson, 2011). Epileptic seizures occur as a result of abnormal electrical discharges from cortical centres in the brain, normally as a result of an alteration to the permeability of a cell's membrane to calcium and sodium (Porth and Matfin, 2009; Gregory and Ward, 2010; Tortora and Derrickson, 2011). In some patients with epilepsy it has been found that neurotransmitter disturbances such as a lack of, or ineffective, g-aminobutyric acid (GABA) may be the cause of the seizures as GABA works within the cerebral cortex as the main postsynaptic inhibitor, but also has been found to act as a presynaptic inhibitor in the spinal cord (Porth and Matfin, 2009; McCance et al, 2010; Cavazos, 2013).
GABA works within the brain and nervous system by binding to two main receptor sites in the neurone's membrane, GABA-A and GABA-B, which mediate the actions of the chloride channels (GABA-A) and potassium channels (GABA-B). This mediates the polarisation and depolarisation of the cell membrane (Cavazos, 2013); therefore, ineffective, or a deficiency of GABA will result in a reduced mediation of the neuronal activity. As a result of this, the cell membrane's polarisation is raised above the normal resting potential, bringing it closer to the threshold potential, the point where an action potential is created (Porth and Matfin, 2009). The raised resting potential creates a graded potential where smaller stimulus can create an action potential, which will then propagate along the neurone. Consequently, there will be a greater number of, and repetitive, action potentials created within the nervous system, causing the abnormal cognitive function and nervous control observed during an epileptic seizure (McCance et al, 2010; Tortora and Derrickson, 2011; Cavazos, 2013).
Clinical manifestation
As these action potentials are transmitted from the cerebral cortex through the brain stem and other accessory bodies, the patient will enter the tonic phase of a seizure. During this stage increased muscle tone and muscle contraction produce rigidity of limbs and abnormal posturing is combined with a loss of consciousness (McCance et al, 2010). It will be common at this stage for the clinician to observe other clinical indicators such as a short period of apnoea associated with the propagation of the abnormal neuronal signals further through the spinal cord (McCance et al, 2010). To combat this, the body will use the natural inhibitory pathways within the brain to prevent the further spread of the neuronal activity; however, this will not inhibit the signals completely immediately. As a result of intermittent inhibition the patient will enter the clonic phase of the seizure where there is alternating contraction and relaxation of muscles, what is observed as the seizure, until there is a complete inhibition of the abnormal electrical discharge within the brain ceasing the seizure (McCance et al, 2010). However, where the inhibitory pathways are overwhelmed or unable to completely end the abnormal electrical activity propagation, the seizure may become prolonged and the patient enters status epilepticus.
Status epilepticus is defined as continuous seizure lasting longer than five minutes or recurrent seizures without periods of consciousness, or lucidity connecting the seizures for 30 minutes or more (Porth and Matfin, 2009; Gregory and Ward 2010; McCance et al, 2010). The risks involved with epileptic seizures are predominantly secondary to the neurological insult. During a seizure there is a 60 percent increase in cerebral oxygen consumption (McCance et al, 2010), and as a result, energy and oxygen stores are rapidly depleted resulting in the need for anaerobic respiration to supply the brain cells. This causes a build up of lactic acid within the brain, resulting in cell destruction (Gregory and Ward 2010; McCance et al, 2010). This is the reasoning behind providing supplemental oxygen to a fitting patient as it helps to prevent further hypoxia and increases the available oxygen within the bloodstream.
| Phase | Physiology | Presentation |
|---|---|---|
| Tonic | Signal propagation through the brain stem increasing muscle tone | Rigidity and loss of consciousness |
| Clonic | Body attempts to inhibit the abnormal signals occurring intermittently | Contraction and relaxation of muscles ‘shaking’ |
| Postictal | Seizure has stopped and body is returning to the baseline | Letharygy, confusion, amnesia |
Physical injury is also common following a seizure with bone fractures and spinal damage a possibility, injuries that clinicians are keen to minimise with rapid an effective cessation of seizures. With associated morbidity from brain damage from prolonged seizure activity it is vital to ensure paramedics are providing fast, safe and effective care to patients who are suffering from a seizure.
Investigations
Although this article does not focus on the full treatment algorithm for a seizure—airway, breathing, etc.—and focuses on pharmacological management, it is important to understand that some baseline observations should be obtained before commencing pharmacological management. This should include the recording of the patient's capillary blood sugar level as patients with profound hypoglycaemia can present with seizures (Gregory and Ward, 2010). This requires rapid intravenous access and intravenous glucose as first-line treatment, as opposed to anti-convulsants.
When assessing a child with a seizure it is imperative to record the patient's temperature, as younger children are susceptible to febrile convulsions due to an inability to regulate their body temperature efficiently (McCance et al, 2010). Although febrile convulsions can present similar to an epileptic seizure they originate from a rapid rise in body temperature and not as a result of a primary neurological issue (Gregory and Ward, 2010; Scanlon and Cook, 2010). Febrile convulsions are usually self limiting and do not typically require controlling with anti-convulsive medication but primarily through the use of antipyretic agents once the seizure has stopped, although if prolonged the patient still may require pharmacological intervention (Scanlon and Cook, 2010).
When assessing any patient with epilepsy as an underlying condition, and not due to an acute episode, i.e. seizure, it is important to consider their psychological welfare and their behavioural state preceding an incident. In this instance it may include gaining a history from friends or relatives surrounding the patient's compliancy with their anti-epileptic medication, if they are prescribed any. It is well known that, especially in the adolescent patient, chronic conditions such as epilepsy can lead to poor self-esteem and further psychological problems (Willmot-Lee, 2008) which may cause the patient to decide become uncompliant with their medication. During an assessment a paramedic should determine if a patient is compliant with their medication as this may be an indicator of the patient's mental state and a potential risk of a seizure.
Pharmacology
Benzodiazepines, as used by paramedics, readily diffuse into cerebral tissues due to their high lipid solubility (Rey et al, 1999; Waller et al, 2010). This gives the drug a high level of bio-availability within the brain, resulting in a rapid pharmacodynamic effect. Benzodiazepines have a selective affinity for the allosteric, or regulatory, receptor site of GABA-A receptors within the nervous system, causing an increased efficacy of GABA. With GABA having greater effect within the brain there is a greater inhibitory effect and a reduction in the amount of action potentials created and transmitted within the nervous system. This results in greater regulation of the neuronal activities (Galbraith et al, 2007; Rang et al, 2007; Waller et al, 2010). The binding of GABA to its receptor site on the cell membrane causes chloride channels to open, creating an influx of the negative chloride ions into the cell and consequently a reduction in the resting cell membrane potential closer to the average resting potential of approximately -90mV in the nervous system (Porth and Matfin, 2009; Waller et al, 2010). This reduces the excitability of the neurons and thus reduces any convulsive activity for the patient. Due to benzodiazepines reducing neurological stimuli, patients may suffer from respiratory depression due to the respiratory centres in the brain becoming affected by the medication. This requires rapid and effective management, including providing positive pressure ventilation via a bag-valve-mask or the administration of antagonistic reversal agents such as flumazenil if an appropriately qualified clinician is available. Furthermore, patients can remain in a sedated state long after the medication has been administered (Joint Formulary Committee, 2013).
Due to the high level of lipid solubility possessed by diazepam it is readily absorbed by the bowel (Waller et al, 2010), this allows for diazepam to have a per-rectum (PR) formulation, which is currently used in a fitting patient where intravenous access is unobtainable or in fitting children (Association of Ambulance Chief Executives (AACE), 2013).
Midazolam is greatly set apart from other benzodiazepines as it benefits from being one of the only water soluble benzodiazepine preparations. As a result, midazolam has the potential to be given by the buccal (see Figure 2) and intranasal route (Joint Formulary Committee, 2013) and being absorbed through the mucous membranes. This article will not discuss the exact pharmacokinetics of intranasal administration; however, it will provide a rationale for its use. In addition, there is a legal aspect to intranasal administration.
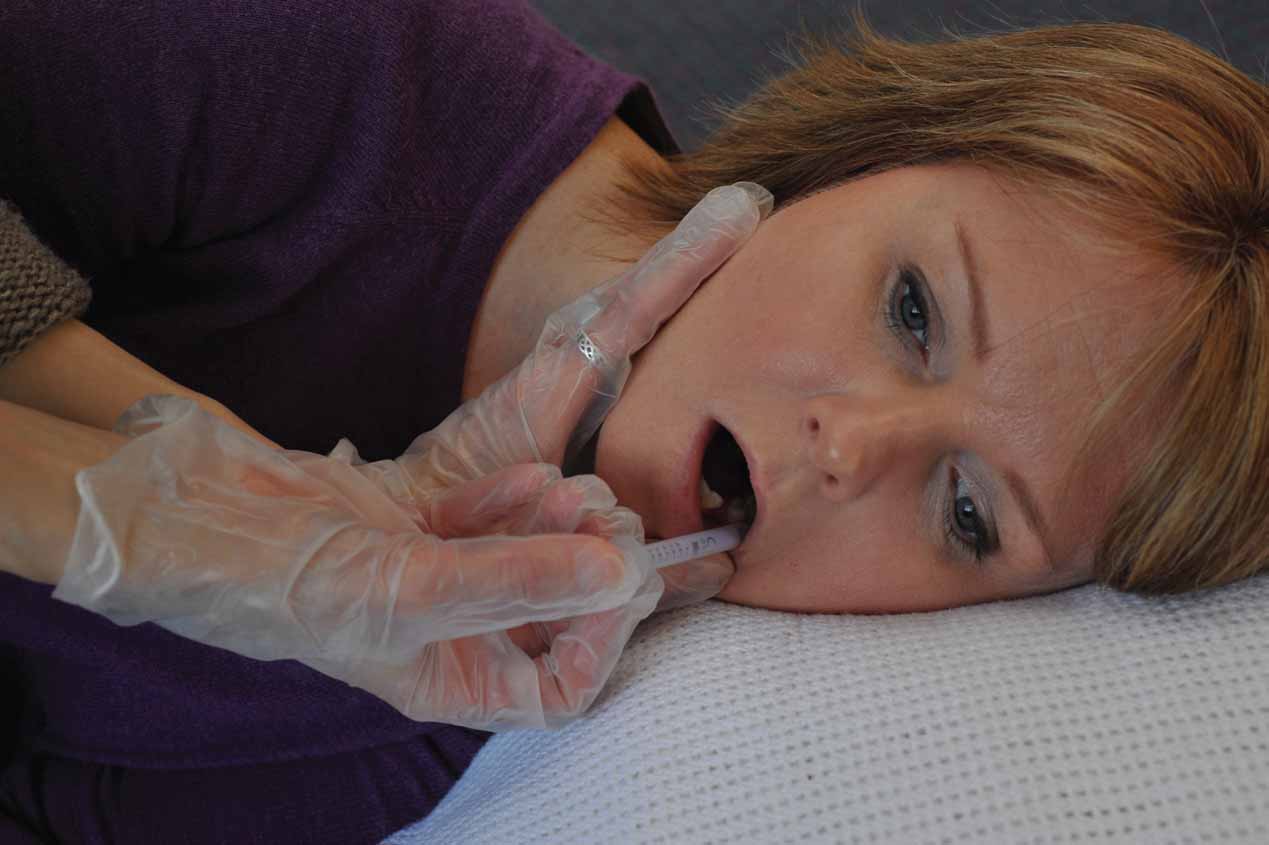
Intranasal and buccal administration of benzodiazepines
Intranasal administration is becoming more widely used for the administration of certain medications by other healthcare professionals (Skuse and Lawlor, 2013), and it is foreseeable that its use would be beneficial to paramedics attending a fitting patient as it negates the need for gaining intravenous access by cannulation. Cannulation carries a high risk of infection to the patient due to it causing a break in the body's natural defences and providing a direct route for infection (Gregory and Mursell, 2010; Lavery 2010). This risk is increased when there is a need for emergency insertion (Gregory and Mursell, 2010), such as a fitting patient, due to aseptic procedures possibly being negated or performed incorrectly. Fitting patients present a high risk of trauma to the patient's veins due to the convulsive activity hindering the paramedic's ability to site the cannula correctly and potentially resulting in an embolus with life-threatening consequences. With infection risk from a needle stick injury being a reasonable possibility (Gregory and Ward, 2010), this may cause concern for many clinicians when attempting to cannulate a fitting patient. It is, therefore, crucial to reduce the incidences where a needle stick injury may occur. These concerns highlight the importance of intranasal and/or buccal administration of medications in patients where there is a high risk of needle stick injury or infection to patients and clinicians alike.
Cannulation is a time-consuming procedure, taking on average approximately three minutes to safely perform the task itself from start to finish (Barrett and Guly, 2000). Further delays arise from decision making and preparation, thus delaying the overall management of a patient and transport to hospital. With a time-critical patient, such as a patient in status epilepticus, rapid cessation of the seizure is imperative and swift transport to hospital needed (McMullan et al, 2010). Cannulation, on average, causes delays on scene of up to 13 minutes (Gregory and Mursell, 2010) and so effective treatment is not being achieved with the current treatment options. Therefore, ambulance services should actively explore alternative treatment options.
In patients where intravenous access cannot be obtained, paramedics can currently administer diazepam rectally. However, this route is not always considered as the first route of administration due to concerns for patients’ dignity, especially if treating a patient in a public area, reservations due to the risk of accusation and difficulty with administration in large or immobile patients (Marshall, 2007). It has been shown in a number of studies and analyses, including a recent Cochrane review (Appleton et al, 2010), that intranasal and buccal administration of midazolam can be used to provide effective and superior seizure control than diazepam administered rectally, and evidence suggests that it can provide successful and greater management than the current intravenous diazemuls (Wolfe and Mcfarlane, 2006; Knake et al, 2009; Appleton et al, 2010; McMullan et al, 2010).
With the evidence suggesting that intranasal and buccal administration of midazolam provides rapid, safe and successful seizure management and understanding cannulation can cause significant delays in patient treatment, it is important to consider if the two drugs are comparable. Due to the ability to administer intranasal midazolam quickly it has been shown to reduce the overall time to seizure cessation in comparison to intravenous diazemuls due to the additional time taken to obtain intravenous access (Appleton et al, 2010; McMullan et al, 2010). This provides a greater benefit to the patient by reducing the overall time they are convulsing and minimising the long-term effects caused by prolonged seizures.
When administering benzodiazepines it is important to be aware of, potentially life-threatening, side effects they can cause. A common side effect of the administration of benzodiazepines is cardiovascular and respiratory depression (Waller et al, 2010; AACE, 2013; Joint Formulary Committee, 2013), which can be life threatening for the patient and is an extreme medical emergency. In sub-anaesthetic doses, as used to treat epilepsy, midazolam benefits from causing little respiratory depression (Rang et al, 2007) in comparison to diazepam which is widely known to cause significant respiratory depression if administered quickly (Joint Formulary Committee, 2013).
Due to diazepam's relative insolubility in water it is normally administered intravenously in the form of an emulsion; however, due to this, injections can be painful for the patient and carry a high inherent risk of thrombophelbitis and venous thrombosis which can manifest days after the initial injection (Joint Formulary Committee, 2013; Waller et al, 2010). Whereas midazolam's water solubility reduces the risk of secondary physical complaints arising from administration.
The legal aspect
Midazolam is a schedule 3 controlled drug (Joint Formulary Committee, 2013). This means that it is a legal requirement to record administration and possession of the drug. The Medicines Act 1968 (c.67) and the Prescription Only Medicines (Human Use) Order 1997 (No.1830) allow paramedics to administer diazepam to patients that clinically require the medication (National Prescribing Centre, 2012). However, until 2012 it has been illegal for paramedics to possess midazolam, although administration was available if a Patient Group Direction was in place. This was until amendments to the Misuse of Drugs Regulations 2001 (No.3998) allowed paramedics, and other healthcare professionals to legally possess and administer midazolam, which has now been partially accepted by the Joint Royal Colleges Ambulance Liaison Committee who have incorporated the use of patients’ own midazolam in their 2013 guidelines (Home Office, 2012; National Prescribing Centre, 2012; AACE 2013). However, this has not been adopted by all ambulance services within the United Kingdom.
Midazolam currently does not hold a licence for use by buccal or intranasal administration, although it is available in this form on prescription only (Joint Formulary Committee, 2013). Due to midazolam currently being unlicensed in any form other than intravenous injection, and paramedics not being included on the list of healthcare professionals able to prescribe unlicensed medications as set out by the Medicines and Healthcare products Regulatory Agency (2009), it will be difficult to implement its complete use in pre-hospital practice without further changes to legislation allowing the administration of intranasal administration under a patient group direction and a manufacturer willing to take these preparations to product licensing to ensure a consistent supply.
It is recommended in the British National Formulary that a healthcare professional who is to administer midazolam should have access to its reversal agent flumazenil (Joint Formulary Committee, 2013). Although the actions of flumazenil will not be discussed in this article it is important to note that clinical guidance suggests its availability when administering midazolam and, currently, flumazenil is unlicensed for paramedic use and therefore further limits the use of midazolam due to the need for further patient group directions or senior medical back up.
Conclusions
With a condition affecting so many people within the United Kingdom it is important to critically analyse the current treatment and management plans used. This article has discussed the pathophysiology of epilepsy and shown how the pharmacology of the medications used results in the reduction and cessation of convulsions. This article has shown the relevant investigations needed when presented with a fitting patient to ensure a correct diagnosis. Although this method is currently unlicensed, there is a clear need for further research into this procedure. With the advent of the new JRCALC 2013 guidelines it is reasonable to assume that the use of pre-hospital midazolam will increase and the drug will become more widely used and commonplace to paramedic practice. However, more research is needed into the routes available for administration of midazolam and further benefits over diazepam in the cessation of seizure activity.

