Cardiovascular disease (CVD) is one of the leading causes of death worldwide. Approximately 17.9 million people die each year as a result of CVD, accounting for 31% of the world's reported deaths (World Health Organization (WHO), 2021). This number may be higher as more than 75% of these deaths are reported in low–middle income countries where the recording of deaths is historically less reliable.
The majority of CVD presentations are as a result of acute coronary syndrome (ACS), which encompasses a plethora of cardiac conditions including angina and myocardial infarction (MI) (Joint Formulary Committee (JFC), 2022). The clinical manifestation of these conditions in the acute setting usually has chest pain as the dominant symptom. Chest pain is a common presentation for patients in the primary care setting with 1.5% of all consultations relating to this complaint (Harskamp et al, 2019).
The task for the assessing clinician is to differentiate between less urgent and more common differential diagnoses of chest pain, such as gastroesophageal reflux disease, musculoskeletal pain and anxiety, and more urgent and less common presentations such as angina, MI and pulmonary embolism.
Accurate and rapid exclusion and identification of ACS is essential if effective evidence-based treatment is to be initiated. Currently, primary care clinicians rely heavily on history taking, with the aid of some diagnostic tools such as electrocardiograms (ECGs) to reach a working diagnosis.
Because of limited access to definitive diagnostic tools, the sensitivity of primary care clinician diagnosis of angina and ACS is around 69% (Harskamp et al, 2019). With the mortality and morbidity risks associated with failure to diagnose ACS, chest pain remains one of the leading causes of referral from primary care to the emergency department (ED), with approximately 700 000 visits to ED each year, equating to 5% of all visits (Goodacre et al, 2005).
Around 18% of patients admitted to the ED with chest pain are discharged with no ACS diagnosis (Nederlandse Zorgautoriteit, 2022). The modern healthcare system has seen increasing demand in recent years with an ever-growing prevalence of chronic disease and an ageing population (WHO, 2021). This places logistical and financial strains on the system, so prehospital clinicians have to ensure referrals are appropriate to prevent unnecessary burden as well as a poor patient journey.

The use of point-of-care troponin testing (POCTT) has been widely explored and debated. The ability of these devices to detect small rises in troponin levels is poor in comparison with that of the high-sensitivity testing equipment in hospitals (Bruins Slot, 2013a; Roffi et al, 2016). This means they are less able to help practitioners make an early diagnosis of MI in patients presenting with recent-onset chest pain.
Because of this, the National Institute for Health and Care Excellence (NICE) recommends the use of high-sensitivity testing as a minimum diagnostic pathway for patients presenting with cardiac-sounding chest pain (JFC, 2022). As such, the uptake of POCTT across the UK remains low. This is mirrored in guidelines from across the world, with Dutch guidelines also recommending against the use of POCTT in its current form (Rutten et al, 2012; Roffi et al, 2016).
In addition, because of the cost and size of current high-sensitivity troponin testing machines, they are not practical for primary care use.
NICE (2018) guidance recommends high-sensitivity troponin testing with a coefficient of variation of ≤10% at the 99th percentile, with the ability to detect cardiac troponin in a minimum of 50% of the reference population. Although the high-sensitivity troponin assays provide near 100% sensitivity, their specificity is low at 60–75% (Vasile and Jaffe, 2018). This burdens the system further with a high rate of false-positive results, which have both financial and logistical implications.
Despite this, since the introduction of high-sensitivity testing, research has confirmed that two-thirds of patients presenting with chest pain can be managed under early-rule-out guidelines and thus receive an expedited treatment pathway, reducing these burdens (Apple, 2009).
As technology improves, so too will the sensitivity of POCTT equipment, with such devices suggested to be currently in development.
In light of this likely improvement, there is a need to collectively review the current research on the use of POCTT in primary care to ascertain its feasibility in reducing unnecessary admissions and improving the diagnosis of ACS. It is unlikely these two matters will depend solely on the sensitivity of the POCTT devices. As such, review of the effectiveness of the devices and assessment of their limitations will play an important role in the decision of whether to implement the devices within primary care settings. Gaps in the research also need to be identified for future studies.
This systematic review aims to collectively evaluate the research relating to POCTT in primary care. The review will consider the diagnostic accuracy of the devices, the processes and how their use impacts on unnecessary hospital admissions and exclusion of ACS. Given the increased focus on health economics and the provision of care that is cost effective, the study will also review subsequent cost implications of the use of POCTT.
Methodology
Search strategy
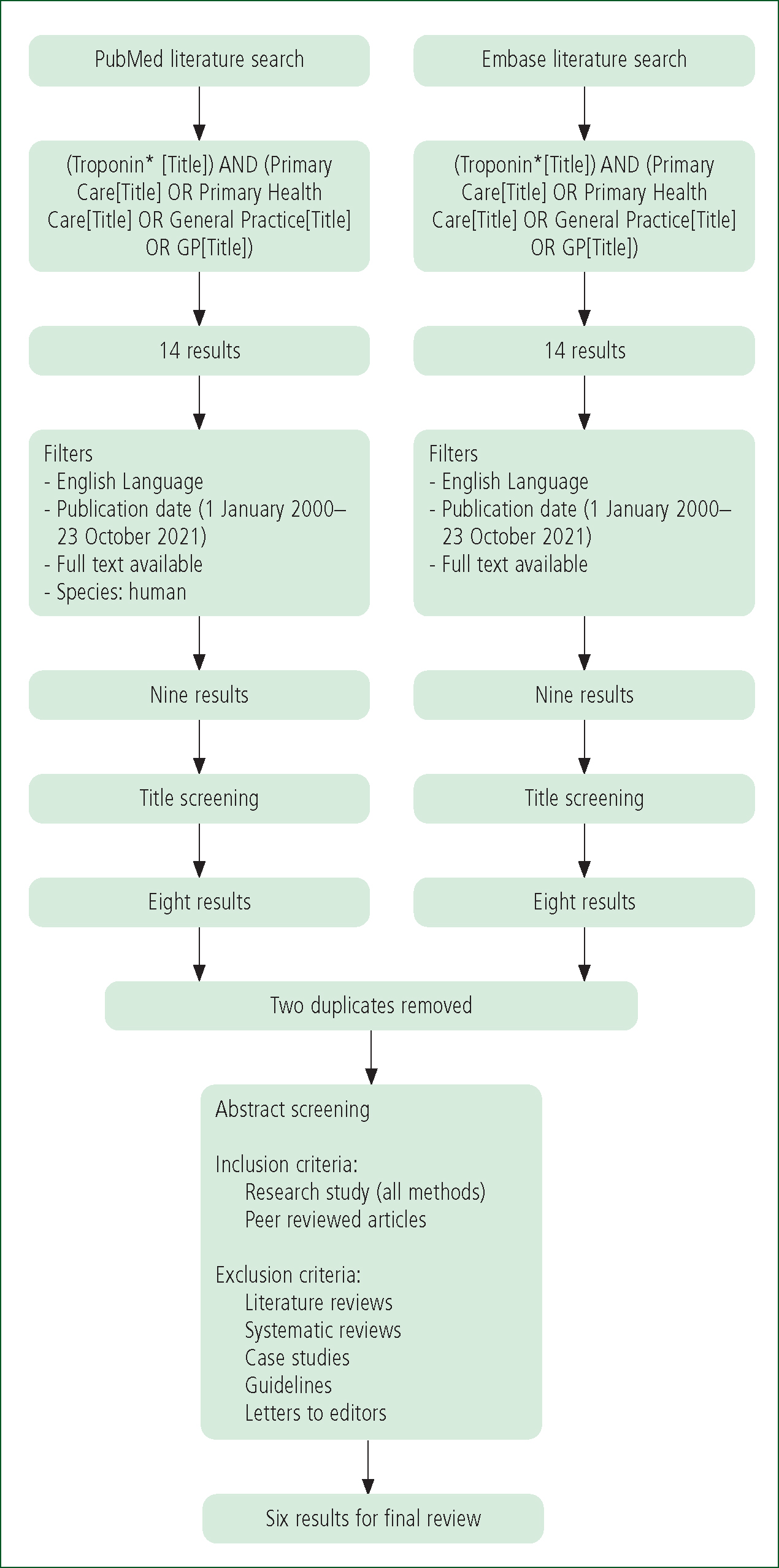
Appropriate MeSH (Medical Subject Headings) terms for inclusion within the literature search were created using the US National Library of Medicine.
The following search terms were included:
Selection criteria
All abstracts of the resulting articles were reviewed for relevance based on the following criteria:
The resulting publications were then reviewed in their entirety to confirm relevance to the research aims and compliance with the inclusion/exclusion criteria. To minimise the likelihood of relevant studies being overlooked, the reference lists of the included studies were reviewed to highlight any pertinent research that fit the criteria but had not been identified from the literature search.
The whole literature search process was then carried out by a secondary researcher to confirm accuracy and compliance and to reduce researcher bias. Following this, no further studies were added and both resulting lists were identical (Figure 1).
Ethical approval
Ethical approval was not required for the completion of this systematic review.
Data extraction
Data were extracted and formatted into tables. They included:
| Study | Summary | Study design and level of evidence | Population, country and time period | Cost of POCTT versus current practice | Change in unnecessary hospital admissions | Acute coronary syndrome exclusion | Mortality | Incorrect diagnosis |
|---|---|---|---|---|---|---|---|---|
| Aldous et al (2012) | An audit of all cardiac troponin testing in an urban community at a single laboratory. Data on admissions and adverse events over a 6-month period was collected on all patients | Audit 4 | 2662 New Zealand 2010 | n/a | n/a | n/a | n/a | 50.79 (1.90%) |
| Nilsson et al (2013) | Comparison of three primary healthcare centres using POCTT compared to centres not testing. Referral rates to ED and diagnosis of MI and unstable angina recorded | Observational, prospective, cross-sectional study with follow-up 3 | 196 Sweden 2010 | n/a | n/a | n/a | Some potential morbidity as two incorrect diagnoses | 2 (1.02%) |
| Nilsson et al (2014) | Comparison of three primary health care centres using POCTT compared to centres not testing. Referral rates to ED and diagnosis of MI and unstable angina recorded and cost implications mapped | Prospective observational 3 | 196 Sweden May 2009–January 2011 | – SKr4538 (£367.72) 10.58% | n/a | +16.08% | Some potential morbidity due to two incorrect diagnoses | 2 (1.02%) |
| Andersson et al (2015) | General practitioner POCTT compared with a high-sensitivity method. 30 days’ follow-up | Observational prospective 3 | 115 Sweden May 2009–January 2011 | n/a | n/a | n/a | n/a Some potential mortality associated due to failure to diagnose AMI | 6 (5.20%) |
| Wilcox et al (2015) | Review of GP-initiated POCTT. Patient outcome data reviewed up to 12 months after test. Clinical information and outcomes were compared with data from emergency department patients with ACS symptoms | Prospective cohort 3 | 396 Australia 24 September 2009 –3 September 2019 | n/a | –8.1% | n/a | n/a | 9.69 (2.4%) |
| Kip et al (2017) | A computerised patient simulation model was used to assess the impact of POCTT on cost of service delivery and testing as well as quality-adjusted life years | Hypothetical cohort 4 | Unknown, Netherlands 2007–2017 | –€77.25 (£65.69) 6.32% | –6.61% | +6.61% | n/a | n/a |
ACS: acute coronary syndrome; AMI: acute myocardial infarction; ED: emergency department; MI: myocardial infarction; POCTT: point-of-care troponin testing
Findings
Data analysis
The quality of the research was assessed using the Oxford Centre for Evidence-Based Medicine Levels of Evidence (Howick et al, 2011). A further review of the heterogeneity of the research ascertained that the research methods varied so a descriptive analysis was performed.
Quality analysis
A quality analysis was completed in line with the Oxford Centre for Evidence-Based Medicine's levels of evidence matrix (Howick et al, 2011). Because of a lack of randomisation and a high use of case control and cohort study methods, the studies received either level three or four.
Patient characteristics were analysed and were broadly similar between the studies (Table 2). Although not all studies recorded age as a mean, their participants show broadly similar characteristics regarding both age and sex.
| Kip et al (2017) | Wilcox et al (2015) | Andersson et al (2015) | Nilsson et al (2014) | Nilsson et al (2013) | Aldous et al (2012) | |
|---|---|---|---|---|---|---|
| Age (years) | No data | 45–74 range | 61–76 mean | 65–66 mean | 65–66 mean | 50–73 range |
| Sex | No data | 55.2% male | 57.4% male | 60.2% male | 57.7% male | 43.1% male |
| Risk factors | No data | Smoker: 23.4% |
Smoker: 13% |
Smoker: 13.8% |
Smoker: 12.7% |
No data |
The risk factor analysis showed a similar percentage of patients with each risk factor.
Cost
Only two studies evaluated cost savings associated with POCTT. Savings ranged from 6.32% (Kip et al, 2017) to 10.58% (Nilsson et al, 2014). Kip et al (2017) reported an average saving of €77.25 (£65.69) per patient, with the current diagnostic pathway costing €1221 (£1038.30) per patient and the POCTT pathway costing on average €1144 (£972.82). Nilsson et al (2014) reported a decrease in SKr4538 (£373.20) between the cost of current practice with no POCTT compared with the use of primary care POCTT.
Unnecessary admission
Kip et al (2017) and Wilcox et al (2015) reported reductions in unnecessary admissions to hospital with the use of POCTT of 6.61% and 8.1% respectively.
With an improved ACS exclusion of 16.08%. reported by Nilsson et al (2014), there was likely some associated reduction in admission; however, an exact figure is not documented.
ACS exclusion
Only Kip et al (2017) and Nilsson et al (2014) referred to the exclusion of ACS as a diagnosis because of POCTT, reporting increased rates of 6.61% and 16.08% respectively. Kip et al's (2017) finding mirrored their figure for avoidance of unnecessary admission.
There is an assumed increase in exclusion of ACS in Wilcox et al (2015) because they reported a decrease in unnecessary admissions. All patients who had ACS excluded were not admitted to the ED.
Mortality
None of the studies reported any mortality associated with POCTT. There may, however, be some associated morbidity given that incorrect diagnoses were reported.
Incorrect diagnosis
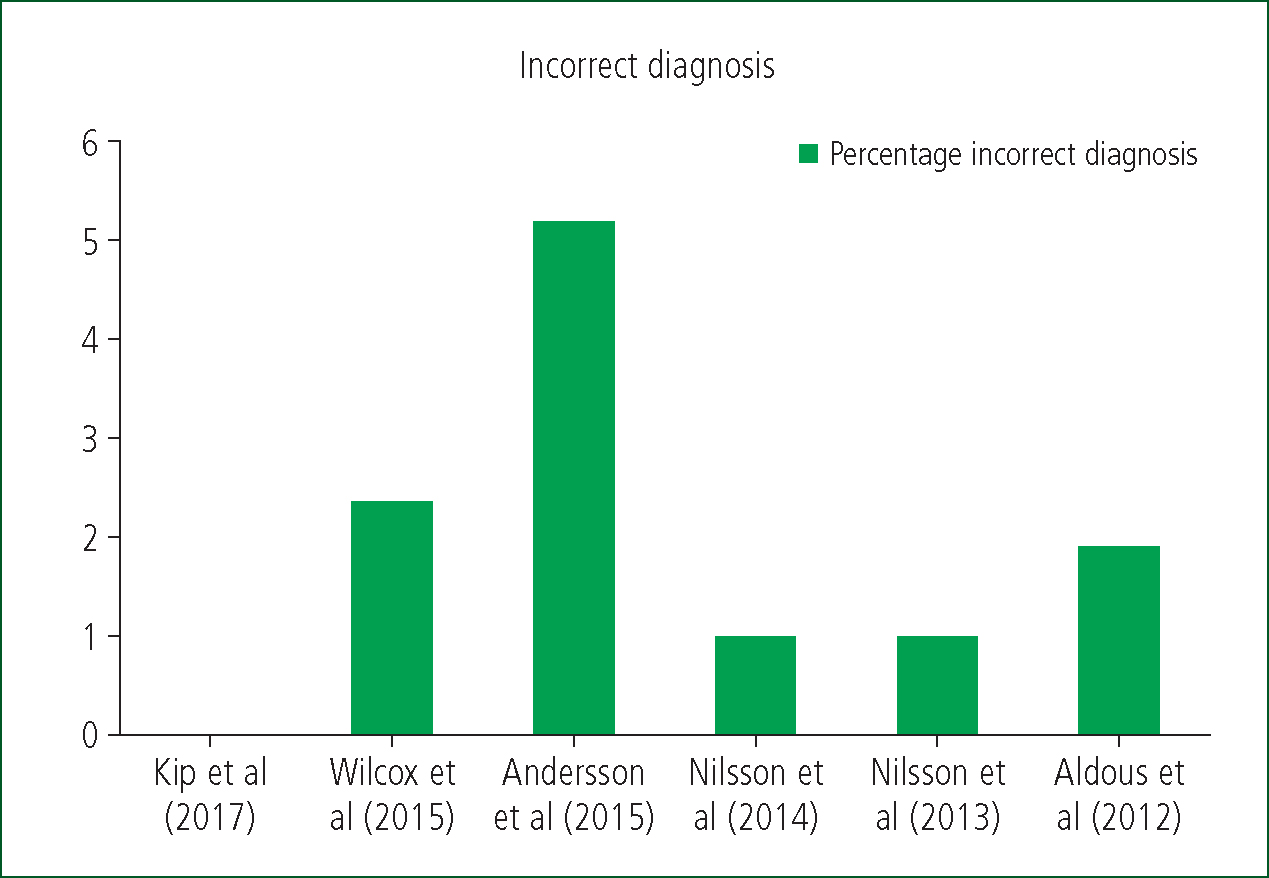
Five out of six of the studies reported misdiagnosis or missed diagnoses despite POCTT use, in a range of 1.02%–5.2%. The highest reported misdiagnosis rate was 5.2% (Andersson et al, 2015). Nilsson et al (2013; 2014) reported from the same research data (Figure 2).
Discussion
Cost
The cost-effectiveness of the healthcare services is an ever-increasing priority for commissioners and providers. The projected NHS healthcare budget for 2020–2021 is £143.3 billion compared to £104.4 billion in 2007–2008 (King's Fund, 2022).
This rise is a direct result of a plethora of pressures on the NHS, such as increased demand associated with the growing prevalence of chronic disease and an ageing population (House of Lords, 2013; Oliver et al, 2014). To remain sustainable, the NHS must balance costs against clinical benefit (NICE, 2013).
Few of the included studies evaluated the cost implications of POCTT, However Kip et al (2017) and Nilsson et al (2014) reported cost savings of 6.32% and 10.58% respectively. Although the savings reported were not significant, the cumulative savings when figures are extrapolated across the nation may result in annual savings of around €14 million (Kip et al, 2017).
These savings could allow finances to be invested into other areas to support the rising costs of healthcare. The final savings may not be significant compared to the overall budget but may lead to a snowballing effect of savings and investment on a wider scale. However, this is heavily reliant on appropriate spending and may not yield results.
Kip et al (2017) emphasised their results were based on conservative estimates so the cost benefit of POCTT may be underestimated. The hypothetical nature of their study, based on a theoretical implementation using a retrospective cohort of patients, resulted in a number of estimates being made that would directly impact cost. Longer times for patient consultations where POCTT was implemented, as well as the number of tests completed, were both estimated. Both could have less of a cost impact than predicted because the costings were based on GPs performing POCTT on all patients with chest pain. The study recognises that this is unlikely to occur in practice so running costs and consultation delays would be lower. Nonetheless, the high number of estimates bring the reliability of the figures into question as, although the estimates may be conservative, without confirmed data they may be underestimations; there may be unforeseen implications, resulting in lower or even no cost savings.
Despite using estimates, Kip et al (2017) did report a reduction in hospital referrals and thus a decrease in the overall cost of €77.25 (£65.69) per patient treatment journey. The study attributed the cost reduction largely to the lower referral rate showing an average reduction of €47.62 (£40.50) per patient against in-hospital treatment.
Nilsson et al (2014) reported a rise in ACS exclusion of 16.08% and, although specific data were not reported, it could be assumed this increase would lead to fewer admissions to ED and thus mirror the cost savings seen in Kip et al (2017).
However, these cost savings were both notably reduced by ACS cases being missed. Although the rate of missed diagnosis was low (1.02% in Nilsson et al (2014)), the potential impact on the overall cost analysis could vary. The morbidity and mortality associated with untreated ACS would pose a lifetime of cost implications which would largely depend on the patient and the severity of the health event.
Nilsson et al (2014) suggest that the overall lifetime costs associated with missed diagnosis may result in POCTT being more expensive than current practices. While these costs are difficult to estimate, Kip et al (2017) adopted a robust approach reviewing cost data from the Dutch Healthcare Authority. This review showed that lifetime health costs in the POCTT group would rise by 6.58%.
Despite missed diagnosis, POCTT still resulted in overall cost savings, as other studies show, which are likely to increase as the sensitivity of the POCTT devices improves (Goodacre et al, 2013). This is reinforced by Nilsson et al (2014), who predicted an overall saving of SKr13 300 (£1085.73) per patient in such improved devices, versus Skr4531.25 (£369.90) per patient with the current POCTT equipment.
POCTT has the potential to have a cost benefit compared to current practice but its associated risk of missed diagnosis would have a varying impact, with the result possibly leading to an overall cost increase.
A theme noted in both Kip et al (2017) and Nilsson et al (2014) is that with an increase in exclusion of ACS, there is a reduction in hospital admission, which will have an overall cost benefit. The extent to which this is reduced by missed diagnosis will depend on the sensitivity of the POCTT devices and the willingness of clinicians to recognise when testing is required and use the device.
If POCTT is to provide a stable and safe financial investment for healthcare systems, high sensitivity is required to prevent increased lifetime costs. It could be argued that current POCTT devices available do not meet this specification so may be a financial risk.
Unnecessary admission and ACS exclusion
Unnecessary admission was directly referred to by both Kip et al (2017) and Wilcox et al (2015) with reductions of 6.61% and 8.1% respectively.
Nilsson et al (2014) reported an increase in ACS exclusion of 16.08%; however, whether this can be directly extrapolated to a reduction in unnecessary admissions is debatable, as it is not known if the clinicians had planned to admit these patients if POCTT had not been available or whether these patients were admitted despite a negative troponin test result. With the results of Nilsson et al (2014) excluded, Kip et al (2017) and Wilcox et al (2015) report broadly similar findings.
The higher avoidance of unnecessary admissions seen by Wilcox et al (2015) may have been a result of their testing methodology. Blood samples were taken in primary care then sent for review in a laboratory. It is likely that the devices used to review the samples are more sensitive than the POCTT devices used in other studies. This methodology demonstrates a unique approach to the use of troponin testing in primary care. Having the samples tested in a laboratory instead of on site, as in the current study, could mitigate the lower diagnosis sensitivity and the risk of misdiagnosis reported in other studies.
However, Wilcox et al (2015) highlighted some key limitations to this approach. The median time from sample to results was reported to be 5 hours. It is likely that this duration would be similar in all cases where this system was adopted because of logistical difficulties behind obtaining the sample and delivering it to an appropriate laboratory for processing. This ultimately led to delays, leaving patients unmonitored in the community awaiting telephone follow-up. Patients with positive troponin results were therefore at greater risk of unrecognised deterioration so this method is not an advisable alternative to current practice.
Despite the apparent benefits of POCTT, Wilcox et al (2015) reported that 13/355 patients were admitted to hospital within 30 days of primary care discharge because of an ACS event. This is in addition to the recorded 2.4% incorrect diagnosis rate. The 13 patients were said to have had negative troponin results so it is difficult to discern whether they had missed diagnoses or isolated later occurrences. This level of misdiagnosis, however, is concurrent with that in the other studies, in a range of 1.02%–5.2%. This pattern increases the suspicion that these were missed diagnoses, possibly as a result of the sensitivity of the testing devices. Wilcox et al (2015) did not document what lab-based analysis equipment was used to test the samples, so comparing the sensitivity to current practice assays is not possible. On face value, the percentage of misdiagnosis is small; however, the risks arguably far outweigh the benefits of rapidly managing patients within primary care.
The POCTT devices are documented to be more accurate at mid-high results (Chapman et al, 2019). Lower results from patients presenting with an ACS event within 4 hours of onset are therefore more likely to be missed. Aldous et al (2012) noted that, of the 21 patients presenting within 10 hours of symptom onset who required serial troponin testing, only 10 had initially elevated troponin levels recorded by POCTT devices. This would suggest that single point-care testing may be appropriate only for a subsection of patients who have been experiencing symptoms for >10 hours. This is in line with other high-powered studies (Mann et al, 2006; Bösner et al, 2009; Bruins Slot et al, 2013b).
Safety netting through patient information leaflets and red flag advice may go some way to protecting these patients. However, early intervention in ACS events reduces morbidity and mortality risks (JFC, 2022), improves the patient's quality of life and reduces their long-term healthcare requirements.
Early recognition is crucial in ensuring the most appropriate diagnostic process is followed. Despite the low percentage of failed diagnoses in POCTT, it may carry enough of a risk to patients and incur such costs that it is not currently viable.
This is further compounded by the lack of misdiagnosis data in the studies comparing ED admission and in-hospital troponin testing to POCTT. For example, Nilsson et al (2013) noted two missed diagnoses because of false negatives versus no missed diagnoses in the other arm. Similar findings have been reported in previous research by Tomonaga et al (2011), with a sensitivity of 100% versus 90% in the POCTT arm.
It could be argued that a patient presenting ≥10 hours since symptom onset with cardiac chest pain would produce a readable troponin result so POCTT devices would be appropriate for these patients. This could mean they may prevent admission of a subgroup of patients and could be used in prehospital services. However, this would first require further study and exploration.
Primary care clinicians have a difficult task. The consequences of misdiagnosis of ACS can be significant morbidity and mortality for patients (NICE, 2014).
With increasing demands on NHS services and the tighter financial controls, primary care clinicians are under pressure to maximise the efficiency of hospital referrals. The risk associated with failed diagnosis may lead to unnecessary investigations and referrals.
The evidence paints a picture of current ACS management practices as being highly sensitive yet with low specificity. Unnecessary tests and referrals are not without consequence. Health risks associated with invasive testing, coupled with the increased stress on the infrastructure, carry a potential risk that may encompass more than the individual involved.
Combining current diagnostic tools to stratify patients’ risk of ACS may go some way to mitigate the limitations of POCTT sensitivity. A combination of POCTT, ECGs, risk factor assessment and patient observations may build a more reliable picture than POCTT alone and reduce the misdiagnosis rate and unnecessary admissions.
Limitations
The limitations of this systematic review largely concern the quality of the included studies. With all studies scoring either three or below on the Oxford Centre for Evidence-Based Medicine Levels of Evidence (Howick et al, 2011) criteria, this would suggest there is a risk of confounding bias and other variables affecting the results. None of the studies used a randomised methodology and future research that took this approach would assist in providing more reliable and robust data.
Diagnostic performance depends on the POCTT equipment used. Various devices are available, with varying performance. Standardisation of devices across the study would have produced more specific and reliable results. However, because studies on the use of POCTT in primary care are limited, this is not possible unless fewer studies are included, which would reduce the population further.
Many of the studies used the same data, which narrowed the field on the resulting population, reducing applicability and thus reliability of the results. All the studies except Aldous et al (2012) had small sample sizes. To improve the reliability of the results to shape future practice, future research could include a larger sample size across multiple sites with a randomised methodology to ensure representative data.
Conclusion
The POCTT devices currently available on the market are not sensitive enough to allow for a robust and reliable diagnosis to be reached. These devices may, however, be used as part of a diagnostic package in combination with ECGs and other diagnostic and risk stratification tools to improve overall diagnostic performance.
It is likely in the near future that the sensitivity of the POCTT devices will improve to a level deemed acceptable by NICE for use in the diagnosis of ACS.
Despite this, the missed diagnosis rates reported in the studies remained low. This would suggest the process carries minimal risk to patients. The morbidity and mortality associated with missed diagnosis, however, may outweigh this and, when devices that are more sensitive become available, they should be used as standard practice.
If high-sensitivity POCTT devices are not possible, then a review should be carried out to assess the results of current POCTT devices used with other diagnostic tools, as well as on patients with a longer symptom duration to see if POCTT may be applicable to this subgroup.

